- Visibility 3.4k Views
- Downloads 1k Downloads
- Permissions
- DOI 10.18231/j.ijpca.2024.045
-
CrossMark
- Citation
A review of isoxazole biological activity and present synthetic techniques
Abstract
Isoxazole compounds have a broad range of biological actions and targets. One of the developments has been the creation of chemicals having heterocycle rings. The incorporation of an isoxazole ring may result in enhanced physical-chemical characteristics. The isoxazole ring is a common moiety in compounds because to its distinct characteristics. design. The primary emphasis of this review article has been on the uses of isoxazole compounds in addressing a variety of illnesses, such as anti-inflammatory, anti-cancer, and antibacterial ones. Compound strategies FDA-approved, preclinical, and clinical medication designs were discussed. Additionally, the focus has been addressed. to the application's future trends and viewpoints.
Introduction of Isoxazole
Isoxazole are major molecules in pharmaceutical chemistry, however many heterocyclics have been studied for the creation of pharmacologically significant chemicals. An oxygen atom sits next to an element of nitrogen in the isoxazole, commonly known as azole. Three carbon atoms make up the ring of isoxazole, an unsaturated aromatic heterocyclic compound. Since the isomer "oxazole" had been discovered first, Hantszch was the researcher who initially proposed isoxazole. The letters "oxa" and "aza" stand for the oxygen atom, "iso" stands for the isomer, and "ole" designates the size of the five-membered ring.
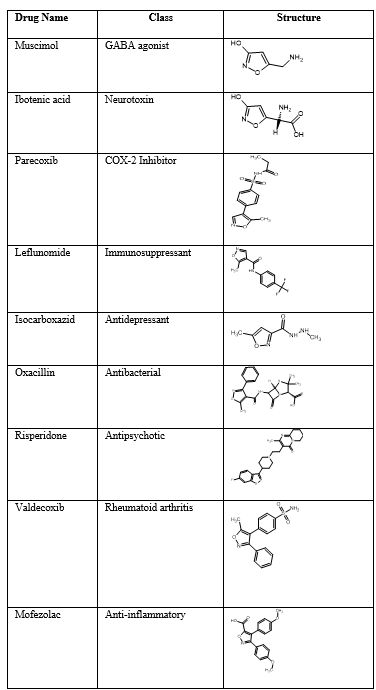
Numerous biological processes are carried out by isoxazole. There is a wide range of variation in the Modification of Isoxazole Structure that is useful for the development of innovative treatments with improved potency and reduced toxicity. The pharma-`cological actions of isoxazole derivatives include analgesic, anticancer, anti-inflammatory, antibacterial, antihistaminic, anti-tubercular, antiulcer, antiepileptic, 5-HTreuptekeinhibitors, antiviral, and anxiolytic properties. The biological action of medications like leflunomide (an antirheumatic medicine) and valdecoxib (a COX-2 inhibitor) rely on the isoxazole ring, which explains the pharmacological benefits of employing this structure.

|
Physical Properties |
|
|
IUPAC Name |
1,2-Oxazole |
|
Molecular Formula |
C3H3NO |
|
Molecular Weight |
69.06 g/mol |
|
Density |
1.074 g/ml |
|
B.P. (Boiling Point) |
95 °C (203 °F; 368 K) |
|
X LogP 3 |
0.1 |
|
H- Bond Donor |
00 |
|
H- Bond Acceptor |
2.0 |
|
State & Colour |
Clear light brown liquid |
Introduction of Chalcone
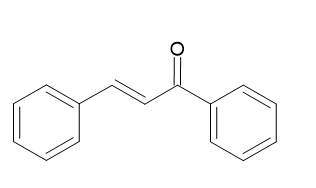
The chemical formula for chalcone is C6H5C(O)CH=CHC6H5. It is an unsaturated ketone. Many different plant species contain large amounts of chalcone. Chalcone are produced by the Claisen-Schmidt condensation procedure. Chalcone is typically generated through acetophenone and benzaldehyde aldol condensation. Chalcone is created in this procedure by mixing acetophenone, aldehyde, and an aqueous sodium hydroxide solution. The chalcones demonstrated a wide range of pharmaceutical effects, including antiproliferative, antiviral, antitubercular, anticancer, antiprotozoal, antioxidant, anti-inflammatory, and antimalarial characteristics.
|
IUPAC name |
(2E)-1,3-Diphenylprop-2-en-1-one |
|
Different names |
“Chalkone” “Benzalacetophenone” “Benzylideneacetophenone” “β-phenylacrylophenone” “γ-oxo-α,γ-diphenyl-α-propylene” “α-phenyl-β-benzoylethylene” “Phenyl styryl ketone” |
|
Molecular Formula |
C15H12O |
|
Molecular Weight |
209.270 g.mol−1 |
|
State & colour |
Solid, Pale yelloe Colour |
|
Density |
1.072 g/cm3 |
|
Melting point (M.P.) |
55 to 57 °C |
|
Boiling Point (B.P.) |
345 to 348 °C |
Methods of Preparation of Isoxazole
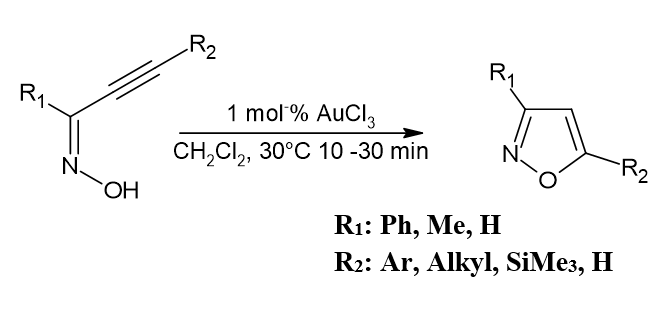
Synthesis of 3-, 5-, or 3,5-disubstitutedisoxazole using AuCl3
Under mild reaction conditions, cycloisomerization of, acetylenicoximes by AuCl3 produces substituted Isoxazole in very good yields. The approach can be used to selectively synthesize isoxazoles that have been 3-, 5-, or 3,5-disubstituted. Corma, A. Antonio Leyva-Pérez et al.[1]
Utilizing the cycloaddition of copper (I , {[1,4-disubstituted 1,2,3-triazoles]} and {3,4-disubstituted isoxazoles} were synthesized
It is simple to get 1,4-disubstituted {[1,2,3-triazoles}] and [3,4-disubstituted isoxazoles}] by cycloaddition cu (Copper) (I) Calcium carbide to azides and nitrile oxides, correspondingly. The process has an extraordinarily wide breadth and high reliability for both of its parts. Computational investigations led to the identification of a non-concerted process involving novel metallacycle intermediates. Himo, Fahmi, et al.[2]
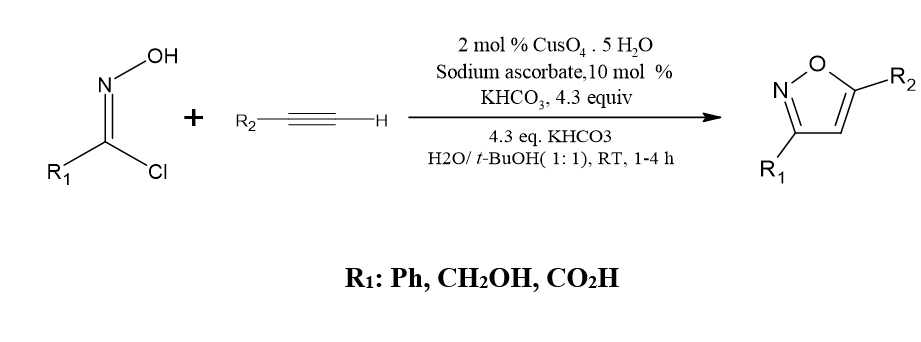
Aldoximes with substituted groups are used to create 3,5disubstituted isoxazoles
Under standard heating conditions 3,5-disubstituted isoxazoles may be effectively synthesized in one pot from substituted aldoximes and alkynes by using either tert-butyl nitrite or isoamyl nitrite. Kadam, Kishorkumar S.et al. [3]
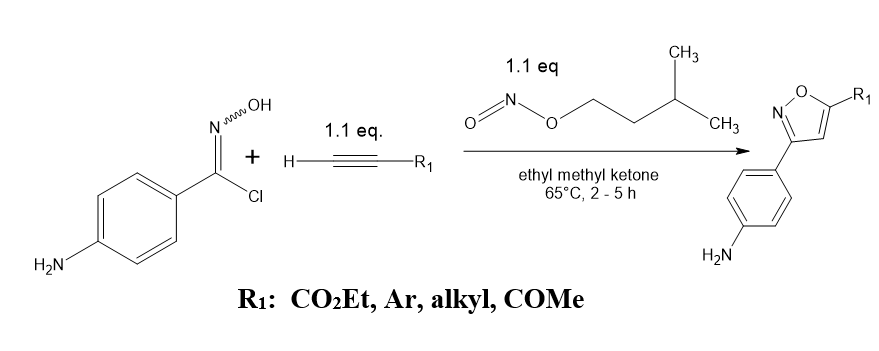
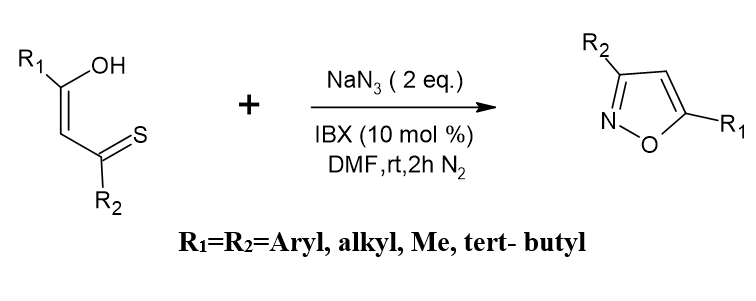
Utilizing sodium azide and 1,3-bis (het aryl monothio-1,3-diketones, 3,5-bis(het aryl isoxazoles are synthesized
Sodium azide and (het) 1,3-bis arylmonothio-1,3-diketones mix well to form large yields in (het) 3,5-bis aryl isoxazoles at room temperature when IBX (iodoxy benzoic acid) acts as a catalyst. Numerous substrates can be employed with the process. When sodium azide and -ketodithio esters react, ketone esters are generated in high yields. Antony P, Mary, et al. [4]
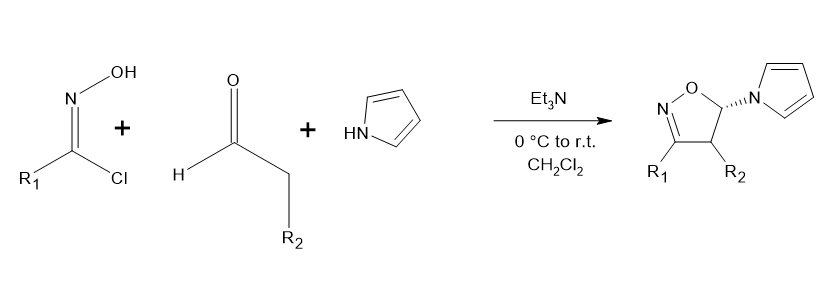
Utilizing [3+2]-cycloaddition to create 3,4-disubstituted isoxazoles
Aldehydes and N-hydroximidoyl chlorides undergo [3+2] cycloaddition reactions in the vicinity of triethylamine, resulting in {[3,4,5-trisubstituted 5-(pyrrolidinyl)-4,5 dihydroisoxazoles]}. Its cycloadducts are subsequently oxidized provides the % of yield is high, region-specific, and free from metal procedure towards the creation of {[“3,4-disubstituted isoxazoles”]} Jia, Qian-fa, et al.[5]
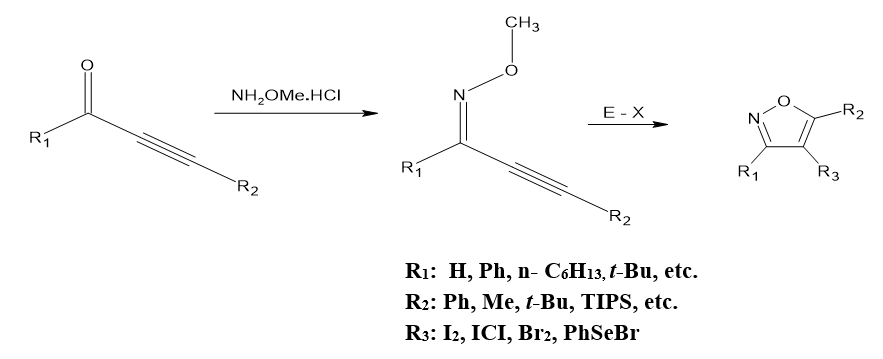
Making 3,5-disubstituted 4-halo(seleno isoxazoles using ICl, I2, Br2/Ph, Se, Br
Under mild reaction conditions, An array of “3,5-disubstituted 4-halo (seleno) isoxazoles” can easily generated excellent to outstanding produces when 2-alkyn-1-one O-methyl oximes are reacted via ICl, I2, Br2, or Ph,Se,Br. Waldo, Jesse P et al.[6]
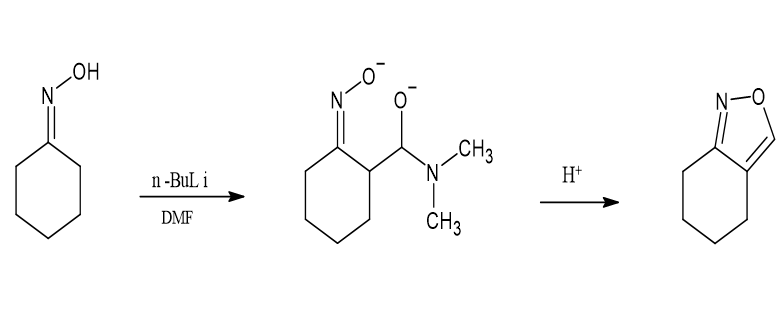
Utilizing l,4-dilithioxime acylation for the synthesis of isoxazoles
It has being cited that acylation of l,4-dilithioximes via amides (DMF)then a mineral acid-infused cyclization dehydration may produce isoxazoles in a very efficient manner. Barber, Gary N., and R. A. Olofson et al. [7]
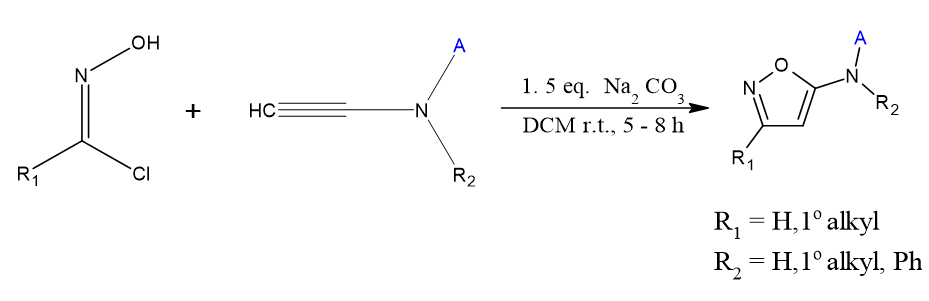
Synthesis of 1,2- disubstituted Isoxazole using [5+1] cycloaddition
1,2 and 3-triazine 1-oxides are produced when tert-butyl nitrite is added to vinyl diazo compounds in their vinylogous state. Since this process, it consists of a formal inter Molecular [5 + 1] cycloaddition, has a significant degree of tolerance for groups of functions and region-selectivity, occurs under favorable circumstances, it may be employed for late-stage fictionalization. When heated to these triazine-N-oxides endure a reaction at the ambient temperature of chlorobenzene, di-nitrogen large yields of isoxazoles are produced via expropriation. De Angelis, Luca, et al.[8]
![Synthesis of 1,2- disubstituted isoxazole using [5+1] cycloaddition](https://s3-us-west-2.amazonaws.com/typeset-prod-media-server/f8a4fcc1-2a6a-4c03-b37d-9da043a4b07bimage10.png)
Synthesis of 5-Substituted 3-Isoxazolols Using Hydroxylamine and α-keto ester
The simplest method to make Isoxazole is by mixing hydroxylamine and α -ketoester. A significant consequence of this cyclization is often the matching 5-isoxazolone.We have discovered that it is possible to create N,O-di Boc-protected –keto hydroxamic acids and cycle them into 5-substituted 3-isoxazolols without the creation of any byproducts. These hydroxamic acid analogues were then converted to the equivalent 5-substituted 3-isoxazolols after being treated with hydrochloric acid. Ulrik S. Sorensen et al.[9]
[Figure 12] Synthesis of 5-substituted 3-isoxazolols using hydroxylamine and α-keto ester
Utilizing Cu (I free cyclization of nitrile oxides to produce Isoxazole)
By first cyclizing nitrile oxides with terminal synamides in the presency of a Cu (I) free catalyst, isoxazoles are created. These isoxazoles may then go through a second cycle formation with internal synamides in the presency of an Au (I) catalyst to create pyrroles. Both of reactions may be carried out in one pot using two procedures. Chen,Changwei, & Sunliang Cui et al. [10]
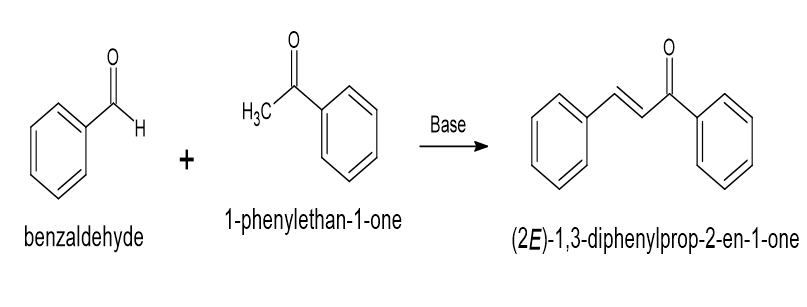
(xi) {displaystyle {ce {C6H5C(CH3)2O2H -> C6H5C(O)CH3 + CH3OH}}} Acetophenone and Benzaldehyde are used in the synthesis of chalcone
Chalcone is generally created by combining benzaldehyde and acetophenone to make an aldol condensation. A 22 gram sodium hydroxide (NaOH) solution in 200 ml of water, 120.5 ml of rectified spirit in a 500 ml head-bolt flask with a magnetic stirrer should be used. While the flask is put in an ice-filled bath and add 0.43 mol acetophenone into it. Put the stirrer on and then add 0.43 mol benzaldehyde. When the mixture reaches the point where stirring is no longer efficient, keep the combination's temperature at around 25oC (the range is 15 to 30oC) and agitate it vigorously for two to three hours. After the stirrer is taken out, the reaction mixture has to be stored overnight in a refrigerator or icebox. For product filtration, use a suction filter or a Buchner funnel. Re-crystallize with ethanol after air-drying. Yazdan S.K. et al.[11]
Biological Activity of Isoxazole
Antibacterial activity
(i)The substituted aromatic ketone and aldehyde are the two molecules were combined with isoxazoles in an effort to produce isoxazoles derivatives [Figure 14] with strong pharmacological activity and little toxicity. When compared to standard drugs tested, certain Isoxazole derivatives showed good antibacterial activity while others showed only moderate activity. Testing of the antibacterial effects was done on both gram-positive and gram-negative microorganisms. The study shown that the antibacterial activity of synthesized Isoxazole derivatives is enhanced by the presence of methoxy, dimethyl amino, and bromine groups at C-5 phenyl rings and nitro, and chlorine groups at C-3 phenyl rings. Chikkula, Krishna Veniet al. [12]
(ii) A novel series of Isoxazole and benzodiazepine derivatives [Figure 15] were made using chalcone, and their capacity to combat bacteria was then examined. First, chalcone were created via Claisen Schmidt condensation of uran-2-carbaldehyde with a variety of acetophenone. Chalcone in ethanol was used to react with hydroxylamine hydrochloride and sodium acetate to produce various Isoxazole derivatives, and it was also used to react with O-phenylene diamine and piperidine to produce different benzodiazepine variations. The selected synthesized compounds' antibacterial potency was evaluated. Walia R, Hedaitullah M et al. [13]
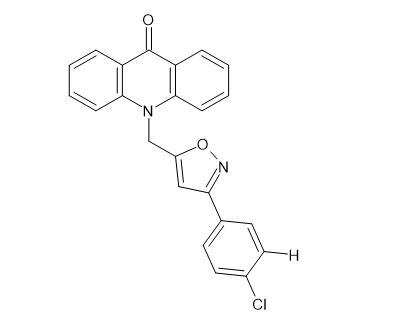
(iii) The test substances demonstrated a range of antibacterial activity when applied against both bacteria Gram(+) and Gram(-). The E. coli strains with the greatest antibacterial activity were discovered. Compound [Figure 16] (10-[3-(4-chlorophenyl)-1,2-oxazol-5-yl] methylacridin9(10H) one) with phenyl and p-nitrophenyl group on the Isoxazole acridone skeleton had the greatest antibacterial activity against E. coli in contrast to the reference medication chloramphenicol (22.41 g/ml). While compound [Figure 16] which contains a hydrogen atom at position C-2 on the acridone ring and a chloro group at position para(p) on the acridone-isoxazole phenyl moiety, has shown remarkable activity against E. coli bacteria (22.39 g/ml). Aarjane, Mohammed, et al. [14]
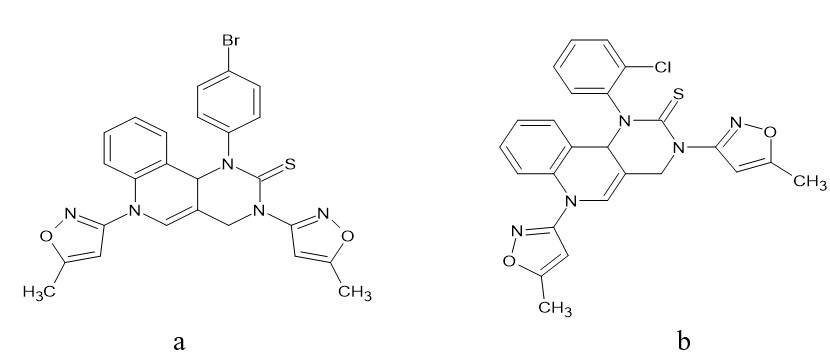
(iv) The activity results demonstrated that these compounds have high antibacterial and antifungal action when compared to conventional drugs. Compounds [Figure 17] and [Figure 17] in particular had the strongest antibacterial activity against all six species (Gramme +ve and -ve) in comparison to the widely used antibiotic Ciprofloxacin. Compounds 4 and 5 were very poisonous to all of the investigated fungi, and even at doses of 100 g/mL, they could kill them all. Zhu, Jie, et al. [15]
Antifungal Activity
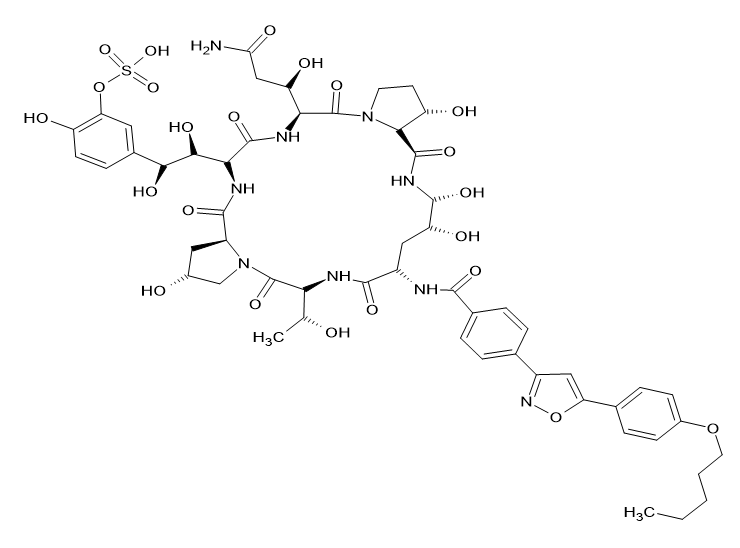
(i) An antifungal drug is a fungicidal or fungistatic substance that is used to treat and prevent mycoses. Adversive and tied to significant mortality as well as morbidity rates are invasive fungal diseases. Additionally, fungi frequently cause superficial infections on the skin and mucosal surfaces. Infected patients' quality of life is noticeably reduced even though it is not life-threatening. Compared to other heterocyclic rings, commercially available antifungal medicines with an Isoxazole ring are unusual. The FDA authorised the echinocandin antifungal medication micafungin on March 16, 2005. It works by preventing the synthesis of 1,3-Beta-D-glucan, which is crucial for the development of fungal cell walls. In Fig-17 the micafungin structure is displayed. Yasuda N. et al.[16]
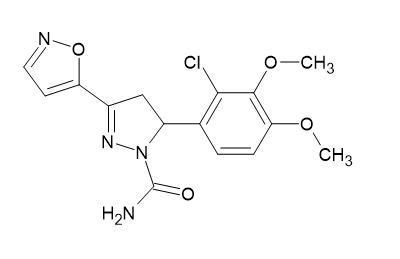
(ii) By using the serial tube dilution method, the target chalcones and pyrazolines [Figure 19] (5-(2-chloro-3,4-dimethoxyphenyl)-3-(1,2-oxazol-5-yl)-4,5-dihydro-1H-pyrazole-1-carbox amide) were examined for their antibacterial properties against two different bacterial and fungal strain types.G+ STP A(Gram-positive Staphylococcus aureus) and G-PS A(Gram-negative Pseudomonas aeruginosa) were the bacterium varieties tested, while Aspergillusniger and Candida tropicalis were the fungal strains. The methoxy group at positions 2 and 4 in the mono substituted chalcone series shown stronger antibacterial and antifungal activity than at positions 3 but less than the standard medicines. Shaik, Afzal, et al. [17]
Antiviral Activity
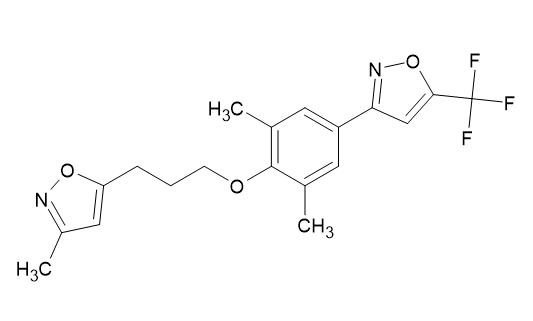
Most antiviral drugs now available target the viral infection hepatitis B and C, herpes viruses, AIDS, and HIV well as influenza A and B viruses since virus requires the host's cells for reproduction. Therefore, researchers are making great efforts to create innovative antiviral drugs that are safer and more effective against a wider variety of viruses. In asthmatic patients who had been exposed to respiratory infections caused by picornavirus, the antiviral medication pleconaril was administered to prevent asthma flare-ups and the symptoms of the common cold. The treatment of infants with enteroviral sepsis was another use for it. Pleconaril's structural breakdown is depicted in [Figure 20]. Naik SM et al. [18]
Analgesic activity
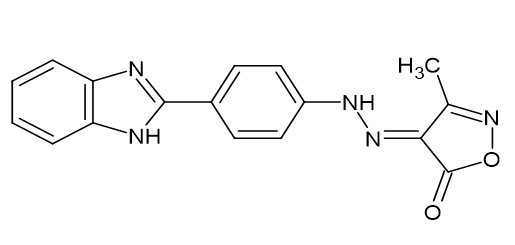
(i) Activity is markedly boosted when the pyrazole's N-1 hydrogen is replaced with a phenyl ring. More substituents were added, such as chloro, fluoro, and methoxy groups, which created very effective analgesic activity at the para position of the phenyl ring. The following para-substituted derivative (4E)-42-[4-(1H-benzimidazol-2-yl) phenyl] Hydra zinyldene-3-methyl-1,2-oxazol-5(4H)-one [Figure 21] demonstrated better or equivalent analgesic activity as compared to conventional Diclofenac. Sahu SK et al.[19]
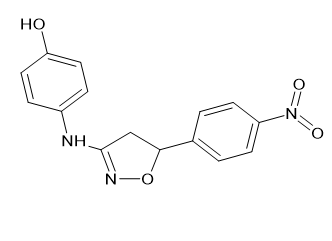
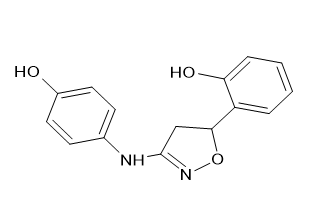
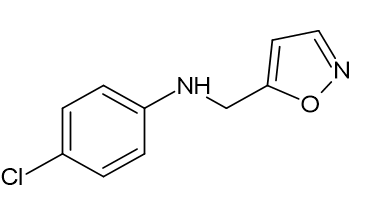
(ii) Either a p-nitrophenyl, p-methoxyphenyl, or p-cholorophenyl 4-{[5-(4-nitrophenyl)-4,5-dihydro-1,2-oxazol-3-yl]2-[3-(4-hydroxyanilino)-4,5-dihydro-1,2-oxazol-5-yl] amino phenol [Figure 22] 4-[5-(4-chlorophenyl)-4,5-dihydro-1,2-oxazol-3-yl] amino phenol [Figure 23], and 1,2-oxazol-3-yl phenol [Figure 24]. The 5-position of the isoxazole ring was modified, which significantly increased the analgesic efficacy. Sahu, S. K., et al. [20]
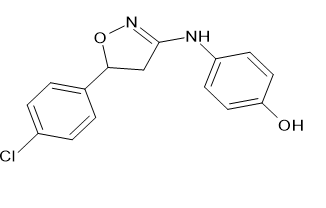
Anti-anxiety activity
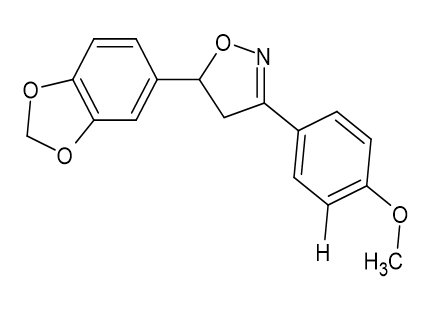
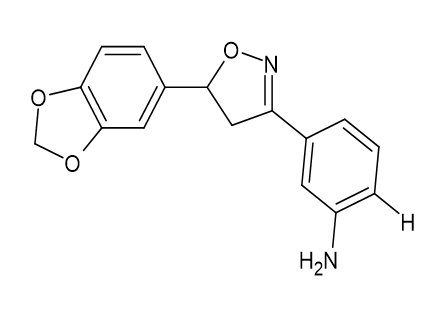
The anxiolytic properties of the proposed analogues 5-(1, 3-benzodioxol-5-yl)-3-(4-methoxy phenyl)-1,2-oxazole [Figure 25] and (3, 5-(1, 3-benzodioxol-5-yl)-4,5-dihydro-1,2-oxazol-3-yl] aniline [Figure 26] were tested in a mirror chamber and raised plus maze apparatus. The acute toxicity research suggested two dosages, with the first dose being 200 mg/kg and the second being 300 mg/kg, respectively, and the second being 231.1 mg/kg and 316.98 mg/kg. Diazepam (2 mg/kg) was used as the standard medicine. 10 ml/kg of 0.5% CMC Sodium were given to the control group. The statistical analysis employed one-way ANOVA, then the Dunnett's t test. P<0.05 was used to determine statistical significance. Mary Sheeja et al.[21]
Anticancer activity
(i) Researchers tested the anticancer potency of a novel family of Isoxazole derivatives known as N-phenyl-5-carboxamidyl Isoxazole using mouse colon carcinoma cells. The findings showed that compound [Figure 27] was the most effective against colon 38 and CT-26 mice colon tumor cells, with an IC-50 of 2.5 g/mL for both cell lines. This suggests that compound [Figure 27] may be further investigated as a viable cytotoxic substance for the treatment of Cancer of the abdomen. Rani P et al. [22]
(ii) The strongest chalcone, first Compound (2E)-1-(1,2-oxazol-5-yl)-3-(2,4,6-trimethoxy phenyl) prop-2-en-1-one was [Figure 28] , at an IC50 of 5.1 g/mL, equivalent to the conventional drug docetaxel. Compound (2E)-1-(1,2-oxazol-5-yl)-3-(2,4,6-tri methoxy phenyl)prop-2-en-1-one, with fluoro at position 2 and -OCH3 at positions 3 and 4, was shown to be the most powerful in the tubstituted series, with an IC-50 of 2 ± 1 g/mL. Zhu J, et al.[23]
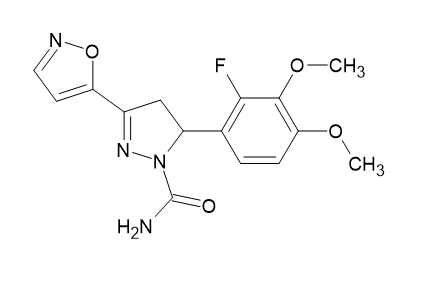
Anti-tuberculosis Activity
To find novel, advantageous anti-TB options, the C-4 position of the 2-amino thiazole core was rationally coupled with several aromatic or heteroaromatic rings. Heterocycles like the Isoxazole ring have exceptional anti-TB effect in contrast to the phenyl ring. N-(5-[2-(4-methyl anilino)-1,3-thiazol-4-yl]-1,2-oxazol-3-yl compound [Figure 29] and 4-[3-(anilinomethyl)-1,2-oxazol-5-yl] are two instances. N-(4 methyl phenyl)-1,3-thiazol-2-amine [Figure 30] was shown to have strong anti-TB activity against susceptible M. tuberculosis strains. They also demonstrated strong efficacy against a panel of resistant strains and selectivity over other bacterial species. Lavanya G, et al.[24]


Anti-inflammatory Activity
Numerous unique Isoxazole compounds were developed as immunosuppressive drugs. Studies on the proliferation of PBMCs (peripheral blood mononuclear cells) induced by periodic health assessments (PHAs) and the production of TNF- induced by LPS in human whole blood cell cultures have been conducted. The compound[Figure 33] was selected as having the greatest potential. Compound [Figure 31] considerably and dose-dependently reduced the inflammation caused by carrageenan in the foot pad, according to the results of the suppressive test. The finding spropose that it could be utilized as a feasible medication for reducing inflammation. Zhu, Jie, et al.[25]

Platelet activation assessed by sP-selectin
The amount of sP-selectin was measured using an ELISA, as directed by the manufacturer (Invitrogen Corporation, Carlsbad, CA). After being thoroughly washed, the platelets were primed for 15 minutes at 37oC with saline, acetyl salicylic acid (0.3mM), or isoxazol2-[5-(cyclohex-1-en-1-yl)-1,2-oxazol-3-yl]pyridine [Figure 32] (0.4-4.4mm) and then activated for 45 minutes at 37oC with thrombin (2 U/ml). The supernatants were then collected after being centrifuged at 2.000 g for 10 minutes at 4oC and they were then kept at 70oC until sPselectin measurements by ELISA could be made. Gutiérrez, Margarita, et al. [26]


Antioxidant Activity
The activity was discovered to be lower than that of gallicacid in general. The data from chalcone and dihydropyrazole can be used to draw the conclusion that the antioxidant activity was found to be enhanced when the electron-donating group (-OCH3) on the phenyl ring was substituted at positions 2, 4, and 6. The most potent molecule in the chalcone series, compound (2E)-1-(1,2-oxazol-5-yl)-3-(2,4,6-tri methoxy phenyl) prop-2-en-1-one [Figure 33] with three methoxy groups at positions 2, 4, and 6, had an IC50 of 5 g/mL and had activity that was equal to the standard. Frølund, Bente, et al.[27]
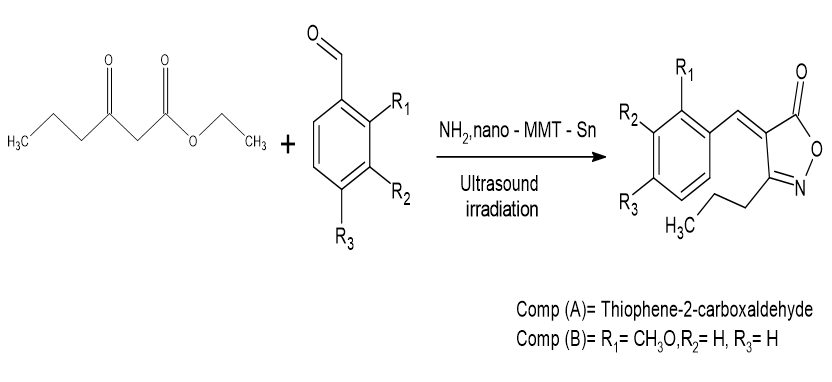
(i) The coupling reaction between aniline derivatives and Isoxazole-Carboxylic acid resulted in the production of a number of isoxazole-Carboxamide derivatives. IR, HRMS, 1H-NMR, and 13C-NMR spectroscopy techniques were used to analyze each of the generated compounds. Hepatocellular carcinoma (Hep-B3 and HepG2), cervical adenocarcinoma (HeLa), breast carcinoma (MCF-7), melanoma (B16 F1), colorectal adenocarcinoma (Caco-2), colon adenocarcinoma (Colo205), and human hepatic carcinoma (HCC) are only a few of the seven cancer cell lines that have been studied. Were tested in-vitro using the MTS assay? For the most efficient chemical, a self-emulsifying method was used to create a nano-emulgel. Hawash, Mohammed, et al.[28]
(ii) With an IC50 of 112.3 1.6 M (%inh = 79.5) and an AC3 of 228 2.3 M (%inh = 68.7) as contrasted to the standard at 18.6 0.5 M (%inh = 87.0), compound (A) had the most promising inhibitory action against (Carbonic anhydrase) CA within the complete series. According to the calculated Gbind (Compounds (A) = 13.53 and (B) = 12.49 kcal/mol), the in vitro enzyme inhibition findings were extensively corroborated by molecular docking (MD) examination, extensive MD simulations (400 ns), and MMPBSA analysis. The in vitro and in silico study also includes a fluorescence-based enzymatic test that showed significant fluorescence amplification for chemicals (A) and (B). Saleem, Afia, et al.[29]
Source of Funding
None.
Conflict of Interest
None.
References
- Corma A, Pérez A, Sabater M. Gold-catalyzed carbon− heteroatom bond-forming reactions. Chem Rev. 2011;111(3):1657-712. [Google Scholar]
- Himo F. Copper (I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J Am Chem Soc. 2005;127(1):210-6. [Google Scholar]
- Kadam K. Alkyl nitrites: novel reagents for one-pot synthesis of 3, 5-disubstituted isoxazoles from aldoximes and alkynes. Synthesis. 2016;48:3996-4008. [Google Scholar]
- Mary A. Reaction of 1, 3-Bis (het) arylmonothio-1, 3-diketones with Sodium Azide: Regioselective Synthesis of 3, 5-Bis (het) arylisoxazoles via Intramolecular N-O Bond Formation. J Organic Chem. 2020;85(23):15422-36. [Google Scholar]
- Jia QF. Synthesis of 3, 4-disubsituted isoxazoles via enamine [3+2] cycloaddition. Synlett. 2013;24:79-84. [Google Scholar]
- Waldo J, Larock R. Synthesis of isoxazoles via electrophilic cyclization. Organic lett. 2005;7(23):5203-5. [Google Scholar]
- Barber G, Olofson R. A useful, regiospecific synthesis of isoxazoles. J Organic Chem. 1978;43:3015-21. [Google Scholar]
- Angelis LD, Zheng H, Perz M, Arman H, Doyle M. Intermolecular [5+ 1]-Cycloaddition between Vinyl Diazo Compounds and tert-Butyl Nitrite to 1, 2, 3-Triazine 1-Oxides and Their Further Transformation to Isoxazoles. Organ Lett. 2021;23(16):6542-6. [Google Scholar]
- Sorensen U, Falch E, Larsen P. A novel route to 5-substituted 3-isoxazolols. Cyclization of N, O-diBoc β-keto hydroxamic acids synthesized via acyl Meldrum's acids. J Organ Chem. 2000;65(4):1003-7. [Google Scholar]
- Chen C, Cui S. Iterative assembly of nitrile oxides and ynamides: synthesis of isoxazoles and pyrroles. J Organic Chem. 2019;84(18):12157-64. [Google Scholar]
- Yazdan S, Sindhura R. Synthesis of substituted isoxazole derivatives from chalcones and their antibacterial activity. Pharmanest. 20141;5(2):1991-4. [Google Scholar]
- Chikkula K, Veni S. Isoxazole-a potent pharmacophore. Int J Pharma Pharm Sci. 2017;9(7):13-24. [Google Scholar]
- Walia R, Hedaitullah M, Naaz S, Iqbal K, Lamba H. Benzimidazole derivatives-an overview. Int J Res Pharm Chem. 2011;1(3):565-74. [Google Scholar]
- Aarjane M. Synthesis and biological evaluation of novel isoxazole derivatives from acridone. Archiv der Pharmazie. 2021;354. [Google Scholar]
- Zhu J, Mo J, Lin Hz, Chen Y, Sun H. The recent progress of isoxazole in medicinal chemistry. Bioorganic Med Chem. 2018;26(12):3065-75. [Google Scholar]
- Yasuda N, Iwagami H, Sasaki Y. Synthesis and antibacterial activity of triazole and isoxazole derivatives of ampicillin. J Antibiotics. 1983;36(11):1516-40. [Google Scholar]
- Shaik A. Antimicrobial, antioxidant, and anticancer activities of some novel isoxazole ring containing chalcone and dihydropyrazole derivatives. Molecules. 2020;25(5). [Google Scholar]
- Naik S, Naik H. Synthesis and antibacterial activity of some chalcones and isoxazoles. Orien J Chem. 1998;14(1):167-75. [Google Scholar]
- Sahu S, Mishra S, Banerjee M, Panda P, Misro P. Synthesis, Partition coefficient and antibacterial activity of 3'-aryl-6'-phenyl (substituted)-cis-5'a, 6'-dihydrospiro. J Indian Chem Soc. 2006;83(7). [Google Scholar]
- Sahu S. Synthesis, analgesic and antimicrobial activities of some novel isoxazole derivatives. Dhaka Univer J Pharm Sci. 2008;7(2):1-6. [Google Scholar]
- Sheeja TM, Mathew A, Varkey J. Design, synthesis and pharmacological evaluation of isoxazole analogues derived from natural piperine. Asian J Pharma Health Sci. 2012;2(1):1-1. [Google Scholar]
- Rani P, Srivastava V, Kurnar A. Synthesis and Biological Evaluation of some Anthranilic Acid and 2-Phenylquinazoline-4(3H)-one Analogues. South Afr J Chem. 2009;62:134-42. [Google Scholar]
- Zhu J, Mo J, Lin H, Chen Y, Sun H. The recent progress of isoxazole in medicinal chemistry. Bioorganic Med Chem. 2018;26(12):3065-75. [Google Scholar]
- Lavanya G, Reddy L, Padmavathi V, Padmaja A. Synthesis and antimicrobial activity of (1, 4-phenylene) bis (arylsulfonylpyrazoles and isoxazoles). Eur J Med Chem. 2014;73:187-94. [Google Scholar]
- Zhu J. The recent progress of isoxazole in medicinal chemistry. Bioorganic Med Chem. 2018;26(24):3065-75. [Google Scholar]
- Gutiérrez M, Amigo J, Fuentes E, Palomo I, Astudillo L. Synthetic isoxazole as antiplatelet agent. Platelets. 2014;25(4):234-8. [Google Scholar]
- Frolund B, Jorgensen A, Tagmose L, Stensbøl T, Vestergaard H, Engblom C. Novel class of potent 4-arylalkyl substituted 3-isoxazolol GABAA antagonists: synthesis, pharmacology, and molecular modeling. J Med Chem. 2002;45(12):2454-68. [Google Scholar]
- Hawash M, Jaradat N, AE, AA, Mufleh O, Al-Hroub Q. Synthesis of novel isoxazole-carboxamide derivatives as promising agents for melanoma and targeted nano-emulgel conjugate for improved cellular permeability. BMC Chem. 2022;16(1). [Google Scholar]
- Ghosh M. . Fundamental of Experimental Pharmacology. 1984. [Google Scholar]
- Abstract
- Introduction of Isoxazole
- Introduction of Chalcone
- Methods of Preparation of Isoxazole
- Synthesis of 3-, 5-, or 3,5-disubstitutedisoxazole using AuCl3
- Utilizing the cycloaddition of copper (I , {[1,4-disubstituted 1,2,3-triazoles]} and {3,4-disubstituted isoxazoles} were synthesized
- Aldoximes with substituted groups are used to create 3,5disubstituted isoxazoles
- Utilizing sodium azide and 1,3-bis (het aryl monothio-1,3-diketones, 3,5-bis(het aryl isoxazoles are synthesized
- Utilizing [3+2]-cycloaddition to create 3,4-disubstituted isoxazoles
- Making 3,5-disubstituted 4-halo(seleno isoxazoles using ICl, I2, Br2/Ph, Se, Br
- Utilizing l,4-dilithioxime acylation for the synthesis of isoxazoles
- Synthesis of 1,2- disubstituted Isoxazole using [5+1] cycloaddition
- Synthesis of 5-Substituted 3-Isoxazolols Using Hydroxylamine and α-keto ester
- Biological Activity of Isoxazole
- Antibacterial activity
- Analgesic activity
- Anti-anxiety activity
- Anticancer activity
- Anti-tuberculosis Activity
- Anti-inflammatory Activity
- Antioxidant Activity
- Source of Funding
- Conflict of Interest
- References
How to Cite This Article
Vancouver
Kumar M, Kumar V, Sharma M. A review of isoxazole biological activity and present synthetic techniques [Internet]. Int J Pharm Chem Anal. 2024 [cited 2025 Oct 28];11(4):307-317. Available from: https://doi.org/10.18231/j.ijpca.2024.045
APA
Kumar, M., Kumar, V., Sharma, M. (2024). A review of isoxazole biological activity and present synthetic techniques. Int J Pharm Chem Anal, 11(4), 307-317. https://doi.org/10.18231/j.ijpca.2024.045
MLA
Kumar, Mohan, Kumar, Vikul, Sharma, Meenakshi. "A review of isoxazole biological activity and present synthetic techniques." Int J Pharm Chem Anal, vol. 11, no. 4, 2024, pp. 307-317. https://doi.org/10.18231/j.ijpca.2024.045
Chicago
Kumar, M., Kumar, V., Sharma, M.. "A review of isoxazole biological activity and present synthetic techniques." Int J Pharm Chem Anal 11, no. 4 (2024): 307-317. https://doi.org/10.18231/j.ijpca.2024.045
