- Visibility 2.2k Views
- Downloads 826 Downloads
- Permissions
- DOI 10.18231/j.ijpca.2024.032
-
CrossMark
- Citation
Comprehensive review on paracetamol: Efficacy, safety, and usage in modern medicine
Abstract
In over-the-counter headache medications, N-acylated aromatic amines—those with an acyl group, RCO-substituted on nitrogen—play a significant role. Drugs that you can get over the counter don't require a prescription. Acetanilide, phenacetin, and paracetamol (acetaminophen) are the so-called "aniline derivatives" or "aniline analgesics," of which paracetamol is essentially the only survivor. Acetanilide is the precursor to both phenacetin and paracetamol. An industrial chemist may refer to paracetamol as 4-hydroxyacetanilide or para-hydroxy acetanilide. It is a crucial end product and precursor for synthesising several other organic chemicals. It has become well-known as an analgesic because of its accessibility, but when overeating or in combination with other foods or medications, it can have severe side effects. This article discusses the historical transformations this significant molecule has undergone up to this point, how chemists prepare it, the processes it goes through when other precursors are present, how much it doses, its adverse effects, and how it is used. This is a beneficial medication that should be handled carefully because it can become poisonous when used with other medicines, including warfarin.
Introduction
Along with aspirin, acetanilide, phenacetin, and acetaminophen are essential ingredients in numerous over-the-counter medications. They are mild analgesics and antipyretics.[1] It was by chance in 1886 that acetanilide's antipyretic properties were discovered. Professor Kussmaul of the Department of Internal Medicine at the University of Strasburg instructed two assistants to cure intestinal worms with naphthalene.
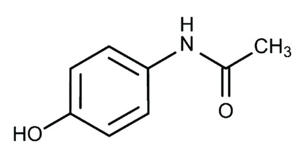
By accident, Cahn and Hepp, who were studying naphthalene as a potential vermifuge (an agent that drives out worms), mixed up a bottle of acetanilide with the naphthalene bottle. The patient's temperature significantly decreased, but his worms did not. Another example of serendipity is that it was so inexpensive to make, and it was put into production very quickly and used for several years. However, it has a dangerous side effect that causes some of the haemoglobin in red blood cells to become inactive. However, because long-term users can harm their kidneys, restrictions have been put on its use. Carl Duisberg, director of research at the Bayer Company in Germany, was interested in Cahn and Hepp's publication detailing their acetanilide tests. Duisberg's issue was how to profitably dispose of approximately 50 tons of p-aminophenol, a by-product of synthesising one of Bayer's other commercial products. He saw right away that adding an acyl group to the nitrogen of p-aminophenol may change it into a substance with a structure resembling that of acetanilide. However, at the time, it was thought that any substance with a hydroxyl group on a benzene ring—phenols, for example—was poisonous. Duisberg created a plan to alter p-aminophenol to produce the chemical phenacetin structurally. It was discovered that phenacetin was a very potent antipyretic and analgesic. An APC tablet, a popular kind of combination pain medication, used to be accessible. Aspirin, phenacetin, and caffeine were the ingredients of an APC tablet (thus, APC). Commercial pain relievers no longer contain phenolacetin. Later research revealed that not all aromatic hydroxyl groups produce harmful chemicals, and acetaminophen, a molecule that was formerly employed in place of phenacetin as an analgesic, is now widely utilised. The over-the-counter pain reliever "Tylenol" is sold as "acetaminophen" (4-acetamidophenol).
Acetaminophen is an over-the-counter analgesic (pain reliever) and an antipyretic (medication to cure fever). Pharmacologically speaking, it is not the same as other over-the-counter drugs like naproxen and ibuprofen, which are similarly used to relieve fever and pain. Acetaminophen comes in pill, liquid, injectable, and rectal suppository forms and is commonly used for pain and fever in both adults and children. Acetaminophen is mainly referred to as "paracetamol" outside the US and Canada, where it is used for the same purposes. [1]
After acetanilide's antipyretic properties were accidentally discovered, it was swiftly adopted into medicine as antifebrin Cahn and Hepp. It was later discovered that acetanilide also has analgesic properties.
However, its intolerable toxic effects—the most concerning of which is methemoglobinemia-related cyanosis—led researchers to look for less hazardous derivatives of aniline. Many substances were put to the test. N-acetyl-p-aminophenol (acetaminophen, paracetamol) and phenacetin (acetophenetidin) proved the most satisfactory. Morse had created the first version of paracetamol in 1878. When von Mering first brought phenacetin and paracetamol into clinical practice in 1887, he quickly abandoned paracetamol in favour of phenacetin because he believed the latter to be less dangerous. Though he mistakenly thought that N-(4-Hydroxyphenyl) ethanamide, also known as paracetamol, produced the same haemoglobin issue as acetanilide, he discovered it was an efficient antipyretic and analgesic. The presence of paracetamol in patients receiving phenacetin doses led to a reexamination of the drug in the 1940s. Sterling-Winthrop Co. began marketing paracetamol in 1953, arguing that it was safer for youngsters and those with ulcers to use than aspirin. On the other hand, prolonged usage damages the liver. [1] When someone takes phenacetin, paracetamol is quickly generated in their stomachs. Since paracetamol is phenacetin's primary metabolite (decomposition product), it is most likely the cause of the drug's antipyretic and analgesic properties. There are claims that the N-oxide, a minor metabolite, caused phenacetin's harmful effects. The breakdown of phenacetin yields two substances. One entails removing the oxygen-bound ethyl (CH3CH2-) group. In the second, a hydroxyl (-OH) group takes the place of the hydrogen atom on nitrogen. We refer to this kind of substance as a hydroxamic acid.
Strong metal ion binding by hydroxyamic acids may be a factor in their toxicity. Though somewhat overshadowed by aspirin, phenacetin was first used in medicine by Dreser in 1899. Since then, it has gained extraordinary popularity and been used without caution, particularly in proprietary analgesic mixtures sold over the counter as "headache mixtures." These mixtures typically contain phenacetin, aspirin, caffeine, and occasionally a barbiturate.
The laity's long-term misuse and abuse of these mixtures, sometimes in enormous quantities over the years, resulted in numerous severe chronic intoxications marked by methemoglobinemia, anaemia, and severe renal damage with a high rate of papillary necrosis (also known as "phenacetin nephropathy" or "analgesic nephropathy"). In 1948, Brodie and Axelrod showed that phenylhydroxylamine, another metabolite, causes methemoglobinemia, whereas paracetamol is the primary metabolite responsible for the analgesic activity of acetanilide and phenacetin.
Paracetamol was thus "rediscovered" and put on the market in the middle of the 1950s. Since around 1980, paracetamol sales have surpassed aspirin in numerous countries, including the United Kingdom, as it quickly acquired popularity. Phenacetin's virtual commercial collapse coincided with this since it was attributed to haematological toxicity, psychotropic side effects, and "analgesic nephropathy," all of which increased the drug's risk of addiction [2].
Alternate names: Acetaminophen, p-Hydroxyacetanilide, p-acetyl aminophenol, Acamol, Abensanil
Chemical formula: C8H9NO2
Appearance: White, odourless crystalline powder; large monoclinic prisms from water
Molecular weight: 151.16
Melting point:169-170.5°C
pH:5.3 to 6.5 at 25°C.
Density 1.293gm/cc
Solubility: Soluble in water (1:70, 1:20 at 100°C), ethanol (1:7), acetone (1:13), chloroform (1:50), glycerol (1:40), methanol (1:10), propylene glycol (1:9) and solutions of alkali hydroxides; insoluble in diethyl ether Slightly soluble in ether. It is insoluble in petroleum ethers, pentane, and benzene.
Stability: Dry, pure paracetamol is stable to 45°C
Dissociation constant: pKa = 9.0-9.5
Partition coefficient Pc = 6.237 (octanol: pH 7.2 buffer)
Chemical Properties of Paracetamol
Preparation of Paracetamol
Phenol formation
Acetaminophen, commonly called paracetamol, is an ordinary painkiller. There are three steps in the process of getting from phenol to acetaminophen. First, phenol is electrophilically aromatically substituted with nitro phenol. The para-substituted nitrophenol's nitro group is reduced to an amine by either direct hydrogenation or sodium borohydride (NaBH4) reduction. Ultimately, acetic anhydride and para-aminophenol react to produce acetaminophen. The nucleophilic amine targets the highly electrophilic carbonyl of the acetic anhydride, much like when an alcohol is esterified by responding with a carboxylic acid. The production of an amide by a reaction with an anhydride has a far more favourable equilibrium constant than the esterification of alcohol by a carboxylic acid because of the strong reactivity of anhydrides and the poor nucleophilicity of the carboxylic acid bi-product. [3]
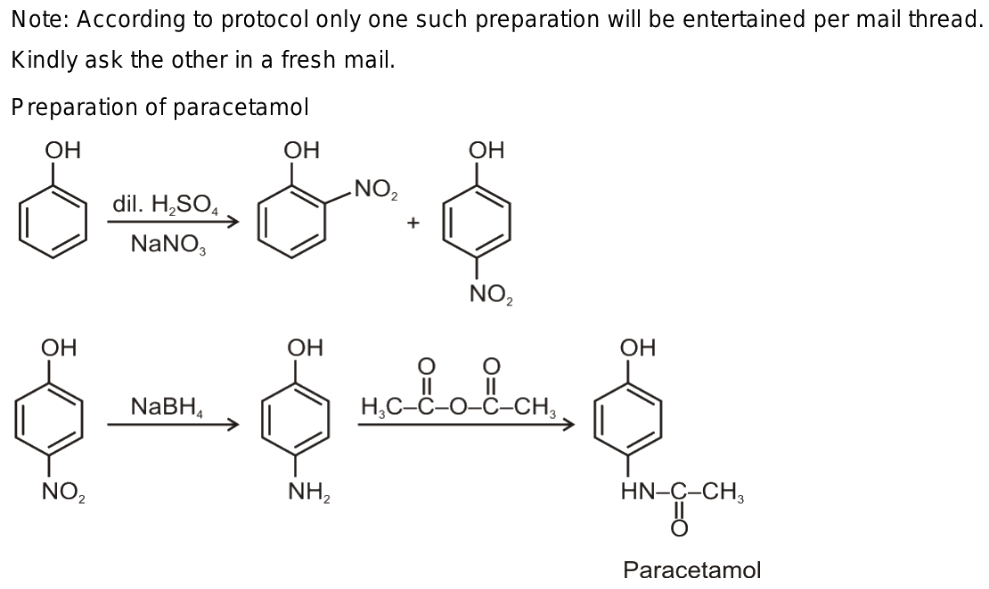
Production of nitrobenzene
When nitrobenzene is electrolytically reduced in sulfuric acid, para-aminophenol is produced. Acetic anhydride and sodium acetate are added to the mixture to produce acetaminophen, also known as paracetamol. [3]
Formation of para-chlorobenzene
Below is a quick description of the PNCB (Para Nitro ChloroBenzene) route procedure.
In an autoclave, caustic soda and para-nitro chloro benzene are reacted for eight hours at 150°C and 5 kg/sq.cm of pressure. Para nitro phenol (PNP), the reaction's byproduct, would be separated using filtration and crystallisation. It would first be treated with acetic acid to transform PNP into para–amino phenol (PAP) to bring its pH down to 3. To make crude paracetamol, the para–amino phenol would be acetylated.The product would undergo additional bleaching using activated carbon to generate snow-white-coloured paracetamol. After that, the product would be dried in a tray drier and milled until it was 40 microns in size.[3]
Clarification of structure
Because it has hydroxyl functional groups, paracetamol (p-hydroxyacetanilide) reacts with phenols as usual. Due to the phenolic group's presence oxidises, undergoes acetylation, and interacts with sodium hydroxide, resulting in a positive iron (iii) chloride test result.[4] Among the other chemical processes, they go through are
4-hydroxy-N-carboxyl alanine synthesis
The product 4-hydroxy-N-carboxyl alanine is created in an oxidation reaction of the amide functional group when paracetamol (p-hydroxy acetanilide) is treated with an acidic dichromate solution.[4] The following is the redox reaction for the reaction:
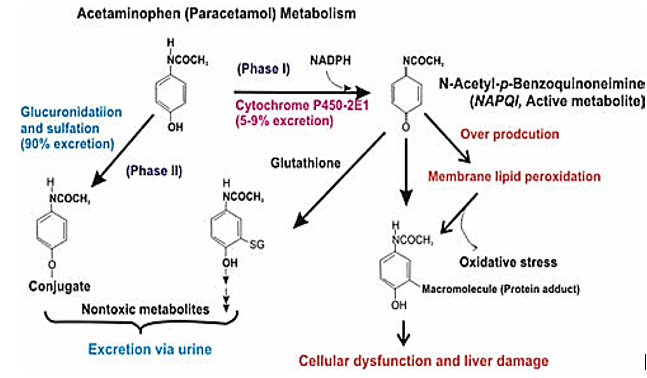
N-acetyl-p-benzoquinoneimine (NAPQI formation)
The production of N-acetyl-p-benzoquinone imine (NAPQI), a hazardous intermediate, occurs when paracetamol is N-oxidized to N-hydroxy paracetamol and then dehydrated.
Ammonium cerium (iv sulphate titration)
To analyse paracetamol, the British Pharmacopoeia procedure calls for heating it under reflux with 1 milligram of sulfuric acid. This is a simple hydrolysis of an amine and a carboxylic acid catalysed by an acid. Ferroin is used as an indication while titrating the generated 4-aminophenol using ammonium cerium (iv) sulfate, an oxidising agent. Ammonium cerium (iv) sulphate's function is to oxidise 4-aminophenol and convert it to iminoquinone. Only when the ferroin indicator changes from Fe2+ to Fe3+ (ferritin) due to the cerium (iv) reagent has all of the 4-aminophenol been oxidised. The solution should be red during titration, and the yellow endpoint is the change from red to pale blue.
Interactions between drugs
The anticoagulant properties of warfarin and acenocoumarol are amplified by paracetamol, which increases the risk of bleeding. Suppressing oral anticoagulant metabolism is one of the proposed mechanisms. However, more current research has yet to support these theories. Patients taking oral anticoagulants should be advised to restrict their consumption of paracetamol. Because carbamazepine stimulates the hepatic metabolism of paracetamol, it raises the risk of paracetamol hepatotoxicity by increasing the development of hazardous metabolites. Similar to carbamazepine, sulfinpyrazone raises the possibility of paracetamol toxicity by promoting the production of hepatotoxic metabolites.
Zidovudine and paracetamol co-administration may cause hepatotoxicity or neutropenia; these side effects haven't always been documented.
The combination with alcohol is a severe cause for concern. The emergence of acute toxic liver symptoms in long-term alcoholics who take paracetamol at doses often regarded as non-toxic is known as alcohol-paracetamol syndrome.
The prognosis for people with alcohol-paracetamol syndrome is worse than that of non-alcoholic patients who overdosed on paracetamol. About 20% of those with alcohol-paracetamol syndrome die overall, and that number rises to over 75% if severe liver failure occurs. Alcohol and paracetamol usage together may accelerate the drug's CYP2E1-mediated metabolism to the extremely hepatotoxic metabolite N-acetyl-p-benzoquinoneimine (NAPQI). Glutathione conjugation detoxifies napqi in non-alcoholics. NAPQI buildup is caused by glutathione depletion and CYP2E1 upregulation in alcoholism. The most significant risk of paracetamol toxicity in these people arises from a brief (12-hour) alcohol abstinence because CYP2E1 is still activated, but alcohol is not present to compete for CYP2E1 metabolism. Furthermore, it has been demonstrated that in epileptic patients using enzyme-inducing anticonvulsants, such as phenytoin and fos-phenytoin, paracetamol has a reduced bioavailability. However, paracetamol improves lamotrigine's excretion through the urine.
Paracetamol Dosage and Adverse Effects
Dosage
It is typically advised to adhere to the dosing recommendations on the package of pharmaceuticals acquired over the counter, even though the maximum daily dosage has generated some controversy in recent years. The child's weight determines most medicine dosages for children. The dosage on the container could be expressed in milligrams (mg) per kilogram (kg) of the child's weight. The recommended dose of acetaminophen for adults and children 12 years of age and older is 650 to 1000 mg every 4 to 6 hours as needed, not to exceed 4000 mg in 24 hours, according to the Tylenol professional product monograph.
The recommended daily dose of acetaminophen for children under 12 is 10 to 15 mg/kg every 4 to 6 hours, with a maximum of 5 doses (50 to 75 mg/kg) in 24 hours. It should be mentioned that there are different liquid acetaminophen concentrations for kids and babies. Therefore, it is highly advised to carefully read the dosage recommendations and contact your child's doctor if you have any queries about how much acetaminophen to give them.[5]
Adverse reaction to acetaminophen
At typical therapeutic dosages, paracetamol has no adverse side effects, such as rash on the skin and other conditions. Now and again, allergic responses happen. Usually erythematous or urticarial, the rash can occasionally be more severe and present with mucosal lesions and medication fever. Seldom do patients who display hypersensitivity to salicylates also show sensitivity to paracetamol and other similar medications. Leukocyte counts may change, although they are usually temporary. The use of paracetamol has been linked in a small number of cases to pancytopenia, thrombocytopenia, agranulocytosis, and neutropenia.
Even in cases of overdosage, paracetamol does not generally produce oxidative hemolysis or methemoglobinemia in humans, but it does in dogs, pigs, and cats. Induced mice have been shown to develop cataracts and exhibit other ocular abnormalities in response to strain; in one study, paracetamol caused a high prevalence of liver cell cancers in 1F mice. Chronically administered high dosages of paracetamol to animals have been linked to spermatogenesis suppression and testicular shrinkage.[6]
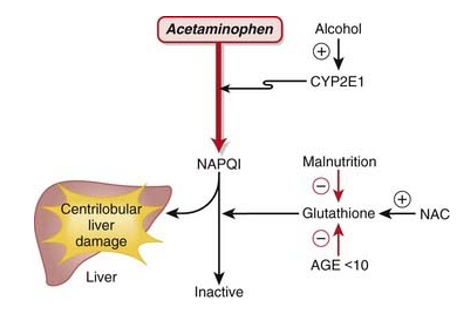
Most people agree that paracetamol is hepatotoxic because cytochrome P450 forms the metabolite NAPQI. CYP2E1 is the most significant of these in terms of numbers.
According to several recent reports, both humans and laboratory animals may have fulminate acute centrilobular hepatic necrosis as a result of a massive overdose of paracetamol. Poisoning is a possibility in cases of overdose, especially in old individuals, small children, patients with liver disease, cases of chronic drunkenness, patients with chronic malnourishment, and patients on enzyme inducers. In some circumstances, poisoning may be lethal. Acute renal tubular necrosis can also result from an acute overdose of paracetamol.
During the first 24 hours, nausea, vomiting, anorexia, pallor, and abdominal discomfort are common symptoms. Overdosing paracetamol (7.5g or more in adults or 140 mg/kg body weight in children) can result in cytolytic hepatitis, which can cause irreversible and complete hepatic necrosis. This can lead to acute or fulminant hepatic failure, hepatocellular insufficiency, metabolic acidosis, and encephalopathy, which can cause a coma or even death.[7]
Use of Paracetamol
In rheumatoid diseases, paracetamol has no anti-inflammatory properties. However, compared to aspirin, it is less harmful and does not cause anaemia and hepatic damage, which can occasionally be brought on by prolonged acetanilide and acetophenotidine use.
The following conditions are treated with paracetamol: common headache relief, muscular and joint pain relief, fever reduction, and relief of cold and flu symptoms.
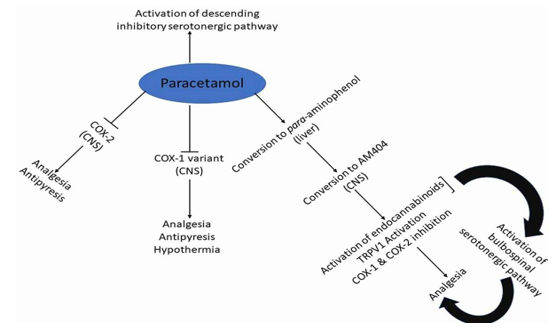
Body Temperature and Fever
The antipyretic properties of paracetamol are widely established. It lowers fever in certain animal species. In rabbits, direct injection into the organum-vasculosum-lamina-terminalis (OVLT) in the front wall of the third intracerebral ventricle revealed a primary location of antipyretic action against fever-induced fever. The fact that paracetamol can reduce a feverish body temperature is less well understood. For instance, several investigations using various delivery systems and dosages have demonstrated that paracetamol causes hypothermia in mice when given intravenously (160 mg/kg, 2.5°C decrease), intraplantarily (100–300 mg/kg, 0.4–2°C decrease), or intracerebrovascularly (dose, 0.25°C decrease). Human data are inconsistent. In patients with subarachnoid haemorrhage or head trauma, a single 1000 mg dose of paracetamol was found to be effective and quick in lowering brain temperature (2°C). In stroke patients, oral or suppository paracetamol administered 6g daily lowered a febrile body temperature by 0.3°C, a decrease linked to a 10%–20% reduction in relative risk. Oral paracetamol (650–1300 mg) has not, however, been shown to lower core body temperature in patients with normothermic heart disease or stroke (3900 mg daily). Therefore, when administered at a therapeutic daily dose of less than 4g, paracetamol-induced hypothermia seems to be clinically inconsequential.
The state of inflammation
Although paracetamol has been shown to suppress several substances linked to inflammation in animals and inflamed dental tissue (1000 mg before treatment and 4000 mg following surgery in patients undergoing two-thirds molar extractions), it is generally not thought to have a particularly potent anti-inflammatory effect in a clinical setting.
In a randomised, double-masked, placebo-controlled trial, for instance, paracetamol is given intraperitoneally (i.p.), orally at 100 mg kg (62), intravenously at 100–300 mg kg or intrathecally at 200 micro g kg, reduced inflammatory pain but did not affect oedema. Additionally, when assessed two and twelve weeks into treatment, no discernible improvement was observed in the paracetamol (1000 mg four times daily) group. One distinguishing feature of paracetamol from NSAIDs is its comparatively weak anti-inflammatory effect, which may be due to a different mode of action.
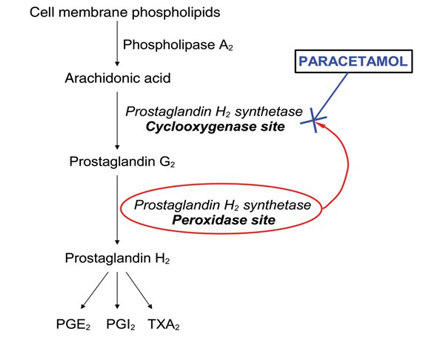
Platelet aggregation
The widespread belief that paracetamol has no clinically significant anti-platelet effect leads to its frequent use as a means of avoiding the bleeding risk connected to aspirin and other NSAIDs. Using in-vitro and ex-vivo assays, there is some indication of paracetamol's anti-platelet activity in human blood samples; nevertheless, other research indicates that the drug has no anti-platelet activity. Unlike the irreversible effects of aspirin and NSAIDs, such an activity is considered reversible (shorter acting) when it occurs. According to at least two recent clinical studies, paracetamol at 1000 mg (73) or 3000 mg intravenously did not prevent platelet aggregation.
On this endpoint, paracetamol may or may not interact with NSAIDs. Another feature that sets paracetamol apart from NSAIDs and may indicate a separate mode of action is its very weak suppression of platelet aggregation.
Conclusion
There are many tales concerning this extremely beneficial yet lethal medication. Some people value its ability to relieve cold and flu symptoms and muscular and joint discomfort; others despise its capacity to cause renal and hepatic problems in humans despite its typical headache, antipyretic, and anti-inflammatory properties. Few people know the chemistry behind the medicine paracetamol despite it being widely used and recognised. The chemical characteristics of paracetamol have been highlighted in this article, and they can be utilised as a starting point to produce new chemicals. This incredibly potent medication's drug interaction is one of its chemical lessons that everyone should learn. It causes severe bleeding when used with other medicines, such as warfarin; patients taking this medication should avoid alcohol. Overdosage can have serious side effects that should not be ignored. It can cause a variety of illnesses, including skin rashes, vomiting, and even damage to the kidneys or liver. Patients should be prescribed the proper dosage and follow it religiously.
Source of Funding
None.
Conflict of Interest
None.
References
- Iwuozor K, Iloamaeke I. Evaluation of the quality of a subset of the Paracetamol tablets available for purchase at the Bridge Head Market in Onitsha. Brit J Res Pharm Med. 2018;3(5):1190-7. [Google Scholar]
- Iwuozor K, Iloamaeke IM. Evaluation of the quality of a subset of the Paracetamol tablets available for purchase at the Bridge Head Market in Onitsha, Anambra state, Nigeria.. CNS Drug Rev. 2006;12(3-4):251-60. [Google Scholar]
- Ashutosh K. A Review on the Properties and Uses of Paracetamol. Int J Pharm Chem. 2004;5(3):83-9. [Google Scholar]
- Frank E. Vanadium geochemistry in the biogeosphere –speciation, solid-solution interactions, and ecotoxicity. Applied Geochem. 2002;102:1-13. [Google Scholar]
- Swarooparani N. A Review on the Properties and Uses of Paracetamol. Int J Chem Phys Sci. 2015;8(4):289-94. [Google Scholar]
- Toussaint K. Melatonin for Sleep Quality and Occupational Cognitive Performance in Shift Workers with Low Sleep Quality: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J Clin Pharm Therap. 2010;35(35):620-6. [Google Scholar]
- Iwuozor K. Evaluation of the quality of a few vitamin C tablets available at Onitsha's Bridge Head Market. J Chem Biomol Eng. 2018;3(3):47-50. [Google Scholar]
- Abstract
- Introduction
- Chemical Properties of Paracetamol
- Preparation of Paracetamol
- Clarification of structure
- 4-hydroxy-N-carboxyl alanine synthesis
- N-acetyl-p-benzoquinoneimine (NAPQI formation)
- Ammonium cerium (iv sulphate titration)
- Interactions between drugs
- Paracetamol Dosage and Adverse Effects
- Use of Paracetamol
- Conclusion
- Source of Funding
- Conflict of Interest
- References
How to Cite This Article
Vancouver
Sharma SK, Tanty KS, Pradhan M. Comprehensive review on paracetamol: Efficacy, safety, and usage in modern medicine [Internet]. Int J Pharm Chem Anal. 2024 [cited 2025 Nov 03];11(3):226-231. Available from: https://doi.org/10.18231/j.ijpca.2024.032
APA
Sharma, S. K., Tanty, K. S., Pradhan, M. (2024). Comprehensive review on paracetamol: Efficacy, safety, and usage in modern medicine. Int J Pharm Chem Anal, 11(3), 226-231. https://doi.org/10.18231/j.ijpca.2024.032
MLA
Sharma, Suraj Kumar, Tanty, Karam Singh, Pradhan, Mukesh. "Comprehensive review on paracetamol: Efficacy, safety, and usage in modern medicine." Int J Pharm Chem Anal, vol. 11, no. 3, 2024, pp. 226-231. https://doi.org/10.18231/j.ijpca.2024.032
Chicago
Sharma, S. K., Tanty, K. S., Pradhan, M.. "Comprehensive review on paracetamol: Efficacy, safety, and usage in modern medicine." Int J Pharm Chem Anal 11, no. 3 (2024): 226-231. https://doi.org/10.18231/j.ijpca.2024.032
