- Visibility 1k Views
- Downloads 82 Downloads
- Permissions
- DOI 10.18231/j.ijpca.2022.027
-
CrossMark
- Citation
Recent advances in sunscreen agents and their formulations: A review
- Author Details:
-
Yamini Shah
-
Rajvee Mewada *
Abstract
Ultraviolet radiation (UVR) has been shown to cause skin disorders, including sunburn and symptoms such as erythema, ageing and formation of wrinkles, pigmentation or dyspigmentation, DNA damage and ultimately photocarcinogenesis on prolonged exposure. It has been reported that sunscreens have beneficial effects in reducing the incidence of skin disorders and protect the skin against exogenous and endogenous harmful agents by absorption, scattering and by blocking phenomena. Ultraviolet (UV) rays are divided into three wavelength categories: UV-A, UV-B and UV-C. Skin exposure to sunlight and other climatic conditions induces the formation of reactive oxygen species (ROS), which can react with DNA, proteins, and fatty acids in the skin, resulting in oxidative damage and damage of the antioxidant system in the human body. Such injuries disrupt the skin’s regulation pathways, resulting in photoaging and the development of skin cancer. Active ingredients in sunscreen agents are synthetic substances which are classified into organic and inorganic filters. Synthetic agents have a number of serious side effects. As a result, to overcome this deleterious effects natural sunscreens were found by the researchers from nature. Natural products can be used as sunscreens and have healing, softening, rejuvenating, and sun protection properties. However, the use of sunscreen has a number of drawbacks, including inducing photoallergic dermatitis, environment pollution, and deficiency of vitamin D production. Therefore, consumers should use appropriate herbal formulations to improve sun protection as well as to avoid the side effects of synthetic sunscreens.
Introduction
Sunscreens are those agents which act by preventing and blocking the damaging effects of ultraviolet (UV) radiation of sunlight.[1] It controls the deleterious effects such as premature aging, which can lead to sagging, wrinkling, hyperplasia associated with UV radiation.[2] Main purpose of applying sunscreen is to protect skin from sunburn, and when looking at the aim by maintaining value of Sun Protection Factors (SPF).[3] These agents are found as over-the-counter products in supermarkets and pharmacies, although they are sold directly by physicians in the USA, in Italy by hospitals, and in Australia by cancer charities and cancer control organizations.[4] In India, the concept of cosmetic is similar to that in the United States, although the list of UV filters is based on the EU model rather than the US model. The Drugs and Cosmetics Act 1940 and Rules 1945 govern cosmetic products in India. Cosmetics standards are determined by the Bureau of Indian Standards (BIS) for products included in Schedule "S" of the Drugs and Cosmetics Rules 1945. UV filters and their products are not included under Schedule S, which means that the Drugs and Cosmetic Rules do not prescribe standard quality of UV filter chemicals or their products in India.[5]
Skin types
The Fitzpatrick scale is a method of classifying skin types based on how they respond to UV rays. The Fitzpatrick scale includes six classifications, skin type I (Always burns easily; never tans; white skin and freckles), skin type II (Burns easily; tans minimally; white skin), skin type III (Burns moderately; tans gradually; fair or beige skin), skin type IV (burns minimally; tans easily; brown skin), skin type V (Rarely burns; tans profusely; darker brown skin) and skin type VI (Never burns; deeply pigmented; black skin).[6] Persons having ‘normal’ skin type are usually classified as Type III. The amount of radiation required to promote melanin synthesis in skin types I and II is far greater than that required to cause an erythemal reaction ([Figure 1] a). As a result, those individuals experience severe sunburn long before the protective melanin layer is formed. For skin type IV and above, the erythemal dose of radiation is much higher than the melanogenic dose, so these types tan well before a reddening reaction is observed. This tanning further increases their protection from erythema. Clearly, the choice of protective formulation made by an individual depends heavily on their skin type; people with type I or II skin need highly protective products, while people with type IV or V skin require only a low level of protection.[7]
Caucasian skin
Fair skinned Caucasians (Skin type I and Skin type II) with lighter skin tones also want to achieve the presumed benefits imparted by even temporary pigmentation. In comparison to their first outdoor exposure in the spring, they detect their skin becoming less vulnerable to acute sunburn ([Figure 1] b) later in the summer.[8]
Asian skin
Asian skin is classified as type IV, which is darker in colour, rarely burns, and is more prone to rapid tanning. Asians have smoother, slightly yellowish skin and are more prone to pigmentation. The presence of the protein melanin in the skin of Asians distinguishes it from the skin of Caucasians. However, in terms of pigmentation ([Figure 1] c), wrinkling ([Figure 1] d), and sunburn, this group demonstrates the impacts of photodamage. Freckle ([Figure 1] e) formation is substantially less common in the Asian population. Overexposure to sunlight, on the other hand, can cause photodamage, including skin cancer. As a result, Asians, like people in other regions of the world, should apply sunscreen on a regular basis as a preventive step. Cosmetic items should be used with caution on Asian skin because it is more prone to hypersensitivity reactions. [9]
Black skin
People with black skin (Skin type V and Skin type VI) are much less susceptible to sunburn than white skinned individuals.[10] On the other hand, they are extremely susceptible to actinic damage and develop precancerous and cancerous lesions ([Figure 1] f) very quickly.[11]
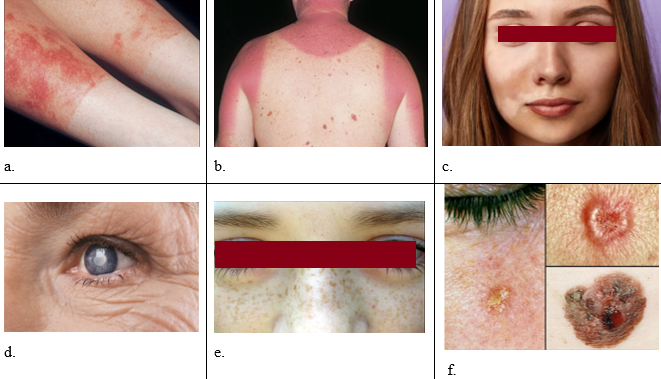
Climatic effects
Studies on skin pigmentation using reflectance techniques have shown that during the summer months skin reflectance decreases, while in the winter it increases, indicating that pigmentation which is increased during the summer has decreased during the winter.[8]
Incoming solar radiation, sun-earth distance, stratospheric temperature, sky condition, total column ozone, altitude, latitude, and solar zenith angle are all parameters that impact the ultraviolet index (UVI), so that it changes from place to place. The annual variation and distribution of the UVI scale during the premonsoon (March, April, May), monsoon (June, July, August), postmonsoon (September, October, November), and winter (December, January, February). Low ozone concentrations after winter and high ozone concentrations after summer may explain the overall general variation in UVI. During the monsoon season, UVI is at its highest. One of the modulating factors of UVI during the monsoon months is cloud cover. As a result, the UVI anomaly is particularly high in July. During the monsoon season, the average UV intensity level across all sites is exceptionally high. As a result, care must be taken throughout the year to prevent the harmful effects of UV-B.[13]
Climatic condition in India
Exposure to UVA and UVB (sunlight) rays is a common occurrence in a tropical country like India, where most regions experience hot to very hot and humid weather. It becomes imperative to take proper precautions to protect the skin from burns and radiation, particularly during the daytime when solar radiation is at its highest.[14] The ambient UV radiation levels in sunlight are greater than in other areas, and most human activities are sunlight-oriented. Sunscreen products are routinely used by people, particularly in urban areas.[15] Sunscreen products claiming an SPF value as high as 40 are marketed as cosmetic products in India.[5]
Types of UV radiations and their effect on skin layers
Sunlight is an electromagnetic radiation that reaches us from the solar surface and spans an enormous range of wavelengths. The UV region of the solar spectrum is directly responsible for tanning and sunburn. The UV region may be further subdivided into UVA radiation between 400 and 320 nm, UVB radiation between 320 and 280 nm, and UVC radiation below 280 nm.[2], [7] UVC has high energy and a shorter wavelength, which is prevented from reaching the earth because it gets absorbed into the ozone layer of the earth’s stratosphere.[16] Due to their high energy, these wavebands are highly cytotoxic to human skin, and although not promoting tanning, they can cause dangerous erythemal reactions or severe skin reddening, even after only brief exposure.[7] UVA (90%-95%) has the highest wavelength and less energy, which can penetrate to the deeper layer of dermis, and it can damage DNA by indirect photosensitizing reaction through the production of ROS.[17] It causes immediate darkening of the skin due to photo-oxidation of bleached melanin, or direct tanning, without any preliminary inflammation and also leads to wrinkling and loss of elasticity due to photochemical breakdown of the fibrous elastic structure and the production of insoluble collagen.[7] UVB (5%-10%) remains in the mid-range, which reaches the epidermis layer and it is absorbed by DNA, which results in molecular rearrangements forming photoproducts like cyclobutene dimer and pyrimidine (6-4) pyrimidine (6-4 photoproducts).[18] This range of wavelengths is the most effective in causing the erythemal reaction known as sunburn, and it can also cause cancerous alterations.[7] ([Figure 2])
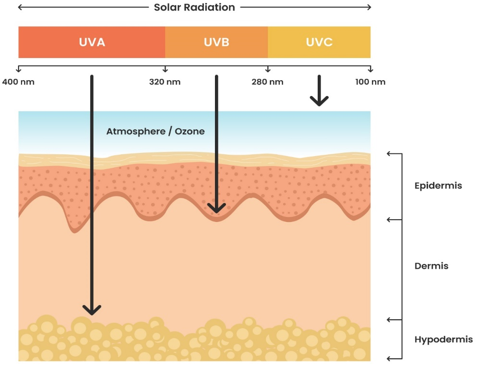
The amount of UVA exposure generally remains the same, whereas UVB exposure occurs more in the summer. Both UVA and UVB radiation are able to cause sunburn, photoaging reactions, erythema and inflammation.[19] It is reported that globally, the application of photoprotective agents has surged in the last few years because of the increase in the occurrence of skin cancer cases, such as squamous and basal cell carcinomas.[20], [21]
Sunscreens
Exposure to UV radiation damages skin function in that UVB affects the upper layer of skin, causing sunburn. Sunscreen agents are used to treat these skin damaging effects.[2] When photoprotective agents are used either therapeutically or prophylactically, the symptoms are improved and the recurrence of the diseases is inhibited, which indicates the need to promote and regularize their application.[9]
Sunlight has many benefits for human health. However, it can also have a detrimental influence.[22] Mitochondrial damage to human dermal cells can also be observed after exposure to artificial sunlight.[23] According to in vitro and in vivo studies, UV-visible irradiation at the boundary region (385-405 nm) can considerably impair skin cells and form dark cyclobutene-pyrimidine dimers.[24] UV-irradiation also plays a direct mutational role in melanoma promotion and oncogene induction, and plays an indirect role through micro-environmental alterations as shown in experimental studies in mice.[25] Therefore, special attention should be paid to protecting human skin from direct exposure to UV irradiation.[22]
Ideal properties of sunscreen
An ideal sunscreen must absorb a broad range of UV rays causing sunburn and be stable in the presence of sunlight to which it is expected to show its efficacy. If the molecule is not stable and gets degraded, which will result into decrease in efficacy or increased toxicity or irritation because of by-products.
It should be able to provide complete protection for the skin against damage from solar radiation, and its ingredients should remain on the upper layers of the skin even after sweating, bathing and swimming.
It should penetrate the skin easily and should not be easily washed away with water or during perspiration.
At low concentration, it should be safe, effective, chemically inert and should not cause irritation, sensitization and toxicity to the skin.[2], [7], [9], [26]
Classification of Sunscreen Agents
Sunscreens provide UV protection in two ways: (a) by preventing free radical formation (Through UV filters) and (b) by scavenging free radicals (via antioxidants).[27] Classification of sunscreen agents are shown in[Figure 3].
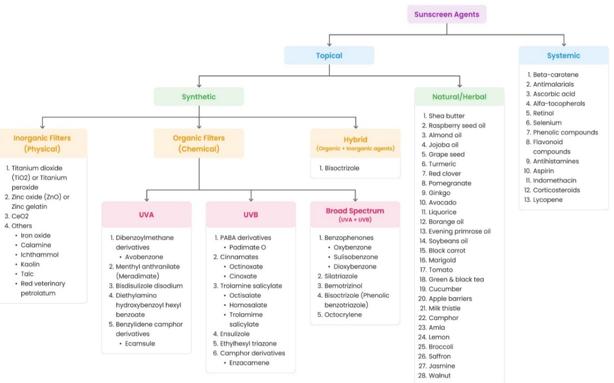
Synthetic UV filters
Inorganic (Physical/Mineral) and organic (chemical) UV filters are two types of synthetic sunscreen agents that have a specific mechanism of action when exposed to sunlight.[28]
Inorganic filters act as a physical barrier for UV radiation. They reflect and scatter UV radiation and protect human skin from direct contact with sunlight, while organic filters absorb specific wavelengths of UV radiation and play an important role in the absorptivity of sunscreen.[29], [30] Sunscreen formulations usually contain both chemical and physical UV filters to increase photoprotection. However, the combination could result in photocatalytic and photolytic processes, causing deterioration of their structure and efficacy.[31]
The USFDA defines broad spectrum sunscreens as those that give UVA protection proportional to UVB protection (FDA-US, 2017).[32] The combination of TiO2 and ZnO ensures broad-band UV protection since TiO2 is more effective at UVB and ZnO is more effective at UVA.[33]
Hybrid materials are made up of two materials that are half-blended together to provide desired functionalities and properties. At the molecular or nanoscale, they are made up of organic (molecule or organic polymer) and inorganic (meal oxides, carbonates, phosphates, chalcogenides, and associated compounds) components. The combination creates ideal materials having a broad spectrum and high chemical, electrochemical, optical transparency, magnetic, and electronic properties. Furthermore, due to their ability to absorb or distribute organic molecules into the hair cuticle and skin layers, several less toxic and biocompatible hybrid materials have been used as active ingredients in cosmetics, hence increasing skin care impact.[28]
Merits
Organic sunscreens
Unlike mineral sunscreens, chemical sunscreens are quick and easy to apply and do not leave a white film on the skin.
Chemical sunscreens perform better statistically on consumers, that look at how long they protect the skin from UVR.[34]
Inorganic sunscreens
The FDA recognizes that titanium dioxide and zinc oxide both as sunscreen chemicals are safe and effective.
Mineral sunscreens are a lot safer for people who are worried about long-term exposure to chemical ingredients.
Mineral sunscreens provide rapid protection without the need to wait 20 to 30 minutes for them to permeate into the skin.
Mineral sunscreens can be applied over makeup and other skin care products.[34]
Demerits
Organic sunscreens
Organic sunscreens are synthetic compounds with a molecular structure that absorbs UV light. Aromatic rings absorb particular wavelengths of light and are commonly seen in these compounds. Over time, the sunscreen loses some of its effectiveness, which causes breakdown of the absorbing molecule. Due to their small size, these molecules may permeate the skin and induce systemic effects. Certain chemical compounds can also behave as haptens and become complete antigens, resulting in sensitization reactions.[5]
Benzophenone-3 (BZ-3) or Oxybenzone is very hazardous, because the chemical is absorbable through the skin and causes endocrine hormone disruption and potentially to cell damage that may lead to skin cancer.[5], [35]
When exposed to sunlight, menthyl anthranilate (meradimate) has the propensity to produce harmful ROS.[5]
Some photo unstable filters (e.g., avobenzone and dibenzoylmethanes) exhibit a variety of photoreactive effects in the formation of photoproducts that can absorb in various UV regions, therefore lowering their photoprotective efficiency.[29] These photodegradation compounds can come into direct contact with the skin, thus promoting phototoxic, photosensitizing, and photoallergic contact dermatitis on the skin.[30]
Para-aminobenzoic acid (PABA) use has declined because of problems with allergic dermatitis and photosensitivity. It is a known carcinogen. Padimate O (Octyl dimethyl PABA) has been proven to cause DNA damage, estrogenic activity, and allergic reactions by releasing free radicals.[36] Ethylhexyl dimethyl PABA is a water-soluble chemical derivative of PABA (4-aminobenzoic acid) that is harmful to the testis, epididymis, spleen, and liver. Furthermore, experiments using human keratinocytes revealed that at low doses, Ethylhexyl dimethyl PABA suppresses cell proliferation and DNA synthesis.[37]
Octinoxate (octyl methoxycinnamate) and Oxybenzone have recently been restricted in Hawaii because of their negative effect on the coral reefs.[38]
Salicylic acid inhibits cholesterol sulfotransferase, which is responsible for the synthesis of cholesterol sulphate in keratinocytes.[39]
4-methylbenzylidene camphor (enzacamene) is an organic camphor derivative that has been considered as an endocrine disruptor with estrogenic activity that could harm human health, particularly in small children, by the general public and the Danish Ministry.[40]
Inorganic sunscreens
Although inorganic agents are generally less toxic, more stable, and safer for humans than organic substances, they can stain clothes due to white pigment residues left on the skin. Since the early 1990s, these metal oxides have been manufactured in the form of micro and nanoscale particles (10–50 nm), which can reduce visible light reflection and make them look transparent across the skin, resulting in improved aesthetics over the larger size.[41] In sunscreen, for example, micro-size TiO2 and ZnO have been substituted with nano-size TiO2 and ZnO, removing unwanted opaqueness and increasing SPF value.[33]
Inorganic zinc oxide (ZnO) and titanium dioxide (TiO2) are used in modern sunscreen formulations because they are highly photocatalytic, have the ability to penetrate the skin, and exhibit potential toxicity.[42]
Sunscreens using TiO2 and ZnO nanoparticles (NPs) tend to block shorter wavelengths from UVAII to UVB rather than longer wavelengths (visible and UVA range). Most NPs can produce ROS radicals when exposed to UV light and are small enough to penetrate the stratum corneum, resulting in severe skin consequences such as photoallergic contact dermatitis and skin ageing with extended exposure. As a result, a variety of parameters, including particle size and distribution, agglomeration and aggregation, and the morphology and structure of nanoparticles, must be managed in order to improve the natural look and prevent skin adverse effects. The use of TiO2 and ZnO NP-coated silicon, or doped elements (Al2O3 and Zr), can reduce ROS formation and prevent the harmful effects.[28], [43]
Natural/Herbal agents
Natural products are produced by living organisms as a secondary metabolite, which possesses antioxidant and UV-absorbing properties.[44] These metabolites are high in antioxidant chemicals and can be employed as inactive substances to protect the skin from harmful effects (e.g., photoaging, wrinkles, and pigment).[28] These antioxidant compounds are obtained from vitamin C, vitamin E, vitamin A, and plant extracts (phenolic, carotenoids, and flavonoid compounds).[45]
Polyphenolic substances like flavonoids and organic UV filters have structural similarities, and they may have photoprotective properties in addition to the antioxidant and absorbance spectrum characteristics of these bioactive chemicals.[46]
Antioxidants are used to neutralize free radicals, and generally a combination of lipid- and water-soluble antioxidants is used to provide complete protection. Antioxidants interfere with the cascade of UV-induced free radical reactions in cosmetic compositions and protect the skin from oxidative stress. Antioxidants are added to sunscreen formulations to provide a second layer of protection, as UV filters alone cannot block 100 percent of UVR from reaching the skin (a product with SPF 50+ blocks 98.3% of UVR).[27]
Merits
Herbal products have fewer side effects on the skin than synthetic products. Herbal sunscreens are non-toxic and non-irritant.[3]
Natural Sunscreen protects the skin from early maturing and it keeps the skin smooth and energetic.[2]
Natural sunscreen is cheaper than synthetic sunscreen, and it is easily available.[2]
They are compatible with all skin types.[2]
Phytoconstituents are gaining prevalence as cosmetic ingredients because they can protect the skin against exogenous and endogenous toxic substances, as well as aid in treating a variety of skin disorders.[47] Herbal extracts have healing, softening, regenerating, and sunblock benefits for these damaged areas.[48]
Because of their different in vivo action mechanisms, polyphenolic compounds have a wide range of pharmacological effects, including antiallergic, anti-inflammatory, hepatoprotective, vasoactive, antithrombotic, antioxidant, free radical scavenging, anticancer, antibacterial, and antiprotozoal.[46]
Demerits
An increase in demand for phytopharmaceutical drugs in the global market may lead to a compromise in quality and quantity due to substitution, adulteration with the wrong plant species, and considerable variation can be found in the content of medicinally active constituents, which will become the greatest threat to the health of consumers.[49], [50]
Adulteration and mis-identification of raw material for phytopharmaceutical drugs are major issues for regulatory authorities, as are the availability and identification of high-quality phytopharmaceuticals.[49]
Expensive in isolation of active ingredients and their methods of standardization.
Complexity in standardizations.[51]
From this, we can conclude that sunscreen made up of natural products is better than the formulation made up of synthetic products. Synthetic products present in formulations are effective, but they have side effects like endometriosis, cytotoxicity, genotoxicity. To overcome this problem, natural products should be used as sunscreen, which has minimal side effects and it is as efficacious as synthetic products.[2]
Examples of herbal sunscreen agents
Butter: Shea butter
Oil: Raspberry seed oil, Almond oil, Jojoba oil, Avocado oil (Persea americana), Borage oil (Borago officinalis), Evening primrose oil (Oenothera biennis), Soybeans oil (Glycine max), Olive oil, Coconut oil, Castor oil, Mustard oil, Chaulmoogra oil, Sesame oil
Rhizomes: Turmeric (Curcuma longa), Broccoli
Flowers: Red clover (Trifolium pratense L.), Marigold (Calendula officinalis), Jasmine (Jasminum officinale)
Fruits: Pomegranate (Punica granatum), Cucumber (Cucumis sativus), Broccoli
Root: Black carrot (Daucus carota)
Seed: Milk thistle (Silybum marianum)
Wood and bark: Camphor (Cinnamomum camphor)
Leaf: Broccoli
Powder: Saffron (Crocus sativus)
Fresh green shells: Walnut (Juglans regia)
Extract
Leaves extract: Green and black tea (Camellia sinensis), Giloy (Tinospora cordifolia)
Seed extract: Grape seed (Vitis vinifera), Ginkgo (Ginkgo biloba), Lemon (Citrus limon)
Root extract: Liquorice (Glycyrrhiza glabra)
Fruit extract: Tomato (Solanum lycopersicum), Apple barriers (Malus domestica), Amla (Emblica officinalis), Lemon (Citrus limon)
Stem bark extract: Arjun tree (Terminalia arjuna)
Literature Review on Herbal Sunscreen Formulations
Arun Rasheed, S. Neelufar Shama, S. Mohanalakshmi and V. Ravichandran prepared sunscreen lotions, possessing broad spectrum of anti-UV radiation effectiveness with reduced concentration of chemical UV filters, from the extracts of bioactive products such as Curcuma longa L. (Zingiberaceae), Aloe vera (Liliaceae) and Alpinia galanga Willd (Siamese ginger). Results showed that the sunscreen lotions were non-mutagenic, non-irritant, stable and possess SPF for normal skin (SPF 55 and SPF 20).[52]
V. Bambal, N. Wyawahare, A. Turaskar and M. Mishra formulated and evaluated the sunscreen activity of Herbal cream containing flower extract of Nyctanthes arbortristis L. (Oleaceae) and Tagetes erecta L. (Compositae). Results showed that N. arbortristis sunscreens will enhance and significantly contribute to the high UV absorbing properties (between 290-320 nm wavelength range) of conventional sunscreen along with the greatest advantage of avoiding the adverse and undesired effects of synthetic sunscreen compounds.[53]
Rohit Marodkar and Dr. Madhuri Pardeshi reported that the herbal extract of cucumber in sunscreen gel works either by absorbing the sun’s UV radiation preventing it from reaching the deeper layers of the skin, or by reflecting radiation and also helps to soothe skin irritation and reduce swelling.[54]
Ruchi Acharya, Anjali Priya, Jaswinder Mehta, Bhawna Sharma, and Peenu Mahendra Joshi prepared a gel from the hydroalcholic extract of Eclipta alba rhizome and these formulations showed good gelling property, washability and homogeneity. All the formulations had a pH that was suitable with the skin’s normal pH range. The gel formulations had a consistent viscosity, neither too thick nor too thin. The Spreadability of the formulation reduced as the viscosity of the formulation increased, and vice versa.[55]
Shweta Kapoor and Swarnlata Saraf evaluated the efficacy of sunscreens containing various herbs such as aloe vera, jojoba, cucumber, wheat germ, olive, and others in protecting skin from UVA and UVB sunrays. The study's findings scientifically confirmed that herbs have the potential to protect skin from damaging sunrays.[56]
Rincon-Fontan M, Rodriguez-Lopez L, Vecino X, Cruz J.M and Moldes A.B prepared a formulations containing Silicate minerals (Mica) and the biosurfactant extract (corn steep liquor) was observed in relation to the protection provided against the UV radiations, as these materials do not exert the harmful effects caused by synthetic organic compounds often added to such formulations, thus providing a new means of obtaining more eco-friendly sunscreen products.[57]
Runa Masuma, Tsutomu Okuno, M Shahabuddin Kabir Choudhur and Takeshi Saito reported that the safety of Tinospora cordifolia and its potential to protect against ultraviolet radiation-induced cytotoxicity and DNA damage in PC12 cells were investigated. T. cordifolia extracts greatly reduced cyclobutane pyrimidine dimer formation induced by UV irradiation at all wavelengths. In conclusion, T. cordifolia is neither poisonous nor harmful to cells.[58]
Manoj Kumar Sahu, Ashish Manigauha and Ram Kumar Sahu formulated and investigated herbal sunscreen cream comprising extracts of plant origin such as Bacopa monnieri, Glycyrrhiza glabra and Daucus carota. The ethanol extracts of Bacopa monnieri, Glycyrrhiza glabra and Daucus carota exhibited the highest total phenolic and flavonoid content. They have shown that these plants shown highest antioxidant and sunscreen activity.[59]
Dinanath Gaikwad and Namdeo jadhav formulated stable emulsified formulations containing Terminalia arjuna (T. arjuna) extract and used in silico molecular screening to determine the antioxidan[60] potential of the final product. According to the results of the solubility study, olive oil, tween 80, and PEG 400 may be the best combination for preparing an emulsified system. The antioxidant potential was discovered by molecular docking via a tyrosinase inhibitory mechanism involving hydrogen bonding interactions. The anti-tyrosinase activity and DPPH free radical scavenging activity of the final product were both good.
Recent Trends in Sunscreen Deliveries
Novel technology has shown considerable potential in terms of increasing the efficacy and efficiency of the delivery of nutraceutical and bioactive ingredients. Nanotechnology has recently shown promise as a potential cosmetic for herbal extracts and phytochemicals that are poorly soluble, poorly absorbed, and labile. The aesthetics and performance of cosmetic products both can be improved with an innovative approach. Novel approaches can also help to improve the efficacy of herbal formulations that have long-term impacts on the human body.[55] The formulation and selection of the final herbal cosmetic products will depend on the purpose of preparation (i.e., for topical or systemic effect; inherent properties of drug or herb extract, such as hydrophilic or hydrophobic; surface characteristics of a system, such as permeability and charges; degree of biodegradability, biocompatibility, and toxicity; release profile and size of the product required; and antigenicity of the final product).[61]
Nano lipid carrier (NLC)
In Nano lipid carriers, the drug is incorporated into the mixture which has a ratio of solid lipids and liquid lipids and it is designed in such a way that it does not form crystals, which is a drawback in Solid Lipid Nanoparticles.[62] Rania et al prepared the oxybenzone loaded NLC gel and found that it provided six to eight times more in vitro SPF and erythemal UVA protection than the free oxybenzone formulation. It has a low potential side effect of skin irritation.[63]
Nano-capsules
Nano-capsules are nanoparticles which are hollow spheres with a diameter of less than 200 nm. It can be filled with either a polar or non-polar solvent.[64] Alvarez et al prepared nano-capsules of Octyl methoxycinnamate with biodegradable polymer and reported that Octyl methoxycinnamate loaded nano-capsules provide protection against UVA induced erythema better than a conventional gel.[65]
Nanoparticles
Nanoparticles are those particles whose size range is 1-100 nm and it is one-dimensional structure.[66] They improved stability of chemically unstable active ingredients. Film formation on the skin allows for controlled release of active ingredients, a pigment effect, and better skin hydration and protection.[61] Marcela et al. prepared a new sunscreen formulation by encapsulating zinc oxide nanoparticles and Octocrylene in polystyrene-co-methyl methacrylate (PMMA/PS) nanoparticles via mini-emulsion polymerization. PMMA nanoparticles were incorporated into the gel. He reported that gel containing PMMA nanoparticles had SPF greater than 30.[67]
Nanosuspension
Nanosuspension is a sub-micron colloidal dispersion having a particle size below 1µm and which is stabilized by surfactant.[68] Villalobos et al. prepared nanosuspension of carnauba wax and titanium dioxide which is distributed into aqueous phase and lipid phase. He reported that SPF value of titanium dioxide nanosuspension distributed in the lipid phase is higher than that of the aqueous phase.[69]
Solid lipid nanoparticles (SLN)
Solid Lipid Nanoparticles comprise of lipid which are dispersed in water or in aqueous surfactant solution. It is a sub-micron colloidal carrier in the size range of 50-100 nm.[70] A solid lipid nanoparticle of Aloe vera was prepared by using a microemulsification technique. Lavita et al. reported that the in vitro SPF of Aloe vera loaded SLN was found to be 16.9± 2.44 and the in vivo SPF was found to be 14.81±3.81 respectively. SLN loaded with Aloe vera was incorporated into a cream and SPF of resultant sunscreen cream is equal to the SPF of sunscreen product available in market.[71]
Nanoemulsion
Nano-emulsions are submicron emulsions in the nano size range.[72] It comprises of two immiscible liquids like water and oil, which are stabilized by surfactant and co-surfactant, reducing the interfacial tension. This is an isotropic dispersion which is thermodynamically and kinetically stable.[73] A nano-emulsion of Rambutan was prepared, which is incorporated into the gel. Muhtadi et al. reported that the SPF of Rambutan nano-emulsion gel was found to be 13.120±0.001.[74]
Microemulsion
They have the ability to encapsulate nonpolar molecules, such as lipids, flavorants, antimicrobials, antioxidants, and vitamins.[61] Microemulsion preparation improves the solubility and stability of the final product. This prepared microemulsion formulation was added to the cream base to develop the final sunscreen formulation with excellent performance in sunburn protection.[75]
Liposomes
Liposomes are small vesicular structures composed of cholesterol and natural, non-toxic phospholipids. The size range is 0.025µm to 2.5µm.[76] Amphiphilic and lipophilic substances, for example, oil soluble UV filters, can be incorporated into the lipid bilayer. Charged but hydrophilic substances can be trapped inside the liposomes. Retains moisture and restore the barrier functions of the skin. Maintain skin appearance by delivering active ingredients to the skin in a continuous release over a long period of time.[61] Haiyang et al. reported that the skin permeation of Quercetin liposomes was 3.8 times higher than Quercetin suspension. It shows the cell viability of UV-B irradiated HaCaT cells increased to 89.89±4.5% for 24 hours and 78.8±3.19% for 48 hours.[77]
Phytosomes
The term ‘Phytosome’ comes from the words “Phyto”, which means “plant” and “some”, which means “cell like”.[78] Phytosomes are lipophilic in nature, which means they promote topical absorption, bioavailability and tissue distribution.[61] Andi et al. reported that a hydrogel containing Propolis phytosome could absorb UV-A and UV-B, with an SPF value greater than 15.[79]
Transferosomes
It can be used for small and large hydrophobic and hydrophilic molecules. Transfersomes have the ability to penetrate the stratum corneum and deliver nutrients to the skin.[61] Kiran S. Avadhani reported that novel nano-transfersomes containing two bioactive molecules, EGCG and HA, were successfully prepared and showed admirable free radical-scavenging effect and negligible cell toxicity.[80]
Regulatory Guidelines for Labelling of Sunscreens
The labelling requirements for sunscreens must adhere to the guidelines issued by the specific regulatory authority. The following information should be included on the label; the identity of the product and the key ingredients, as well as excipients and their percentage composition. The list of ingredients should be in ascending order of predominance from the highest to the lowest. A statement with the name and location of the manufacturer or distributor of the product, cautionary warnings in case a patient is allergic to any of the formulation constituents, optimal storage conditions, appropriate use, frequency, SPF value, and water resistance should all be included on the label.
The EU cosmetic regulations require certain ingredients such as nanomaterials to be included in the sunscreen products labelling.
The EU and the Australia Therapeutics Goods Agency (TGA) do require the inclusion of the product’s shelf life in the packaging label.
In the USA, some sunscreen products do not require expiration dates. This is in circumstances where the manufacturer provides documented evidence that the product has been stable for at least three years. (FDA, 2018b).
The FDA does require the labelling to include the expiration date for sunscreens where the manufacturer cannot provide the stability data to this effect.
In the year 2019, a monograph on sunscreen regulation will comes into effect. (FDA, 2018b, 1999).[81]
Abbreviations
UVR: Ultraviolet Radiation
UV: Ultraviolet
DNA: Deoxyribonucleic acid
ROS: Reactive oxygen species
SPF: Sun Protection Factor
UVI: Ultraviolet Index
Source of Funding
None.
Conflict of Interest
None.
References
- Yadav H, Kasina S, Sunscreens R. . Nanobiomaterials in Galenic Formulations and Cosmetics: Applications of Nanobiomaterials. 2016;1:201-30. [Google Scholar]
- Bhattacharjee D, Preethi S, Amit B, Patil V. A comparison of Natural and Synthetic Sunscreen Agents: A Review. Int J Pharm Res. 2021;13(1):3495-595. [Google Scholar]
- Mansuri R, Diwan A, Kumar H, Dangwal K, Yadav D. Potential of Natural Compounds as Sunscreen Agents. Pharmacogn Rev. 2021;15(29):47-56. [Google Scholar]
- Diffey B. Sunscreens : use and misuse. Comprehensive Series in Photosciences.. 2001;3:523-34. [Google Scholar]
- Kumar S, Gupta R. Safety and regulatory issues on sunscreen products in India. Sch Res Libr. 2013;5(2):145-53. [Google Scholar]
- Fitzpatrick T. The Validity and Practicality of Sun-Reactive Skin Types I through VI. Arch Dermatol. 2013;124(6):869-71. [Google Scholar]
- Ashley L. . Sunburn and sunscreen preparations. 1993. [Google Scholar] [Crossref]
- Sayre R, Ph D, Desrochers D, Wilson C, Ph D, Marlowe E. Skin type , minimal erythema dose ( MED ), and sunlight acclimatization. J Am Acad Dermatol. 1981;5(4):439-82. [Google Scholar]
- Latha M, Martis J, Shobha V, Shinde R, Bangera S, Krishnankutty B. Prabhakar Rao B r. NK. Sunscreening Agents. J Clin Aesthet Dermatol. 2013;6(1):16-26. [Google Scholar]
- Diffey B, Fajuyigbe D, Wright C. Sunburn and sun protection in black skin. Int J Dermatol. 2019;58(9):1053-5. [Google Scholar]
- Kaidbey K, Agin P, Sayre R, Ph D, Kligman A, Ph D. Photoprotection by melanin-a comparison of black and caucasian skin. J Am Acad Dermatol. 1979;1(3):249-60. [Google Scholar]
- . Skin cancer Interactive Medical Media LLC, ISM / Phototake).. . 2007. [Google Scholar]
- Bhattacharya R. Annual Variability and Distribution Of Ultraviolet Index Over India Using Temis Data. Int J Eng Sci Technol. 2012;4(11):4577-83. [Google Scholar]
- . Sunscreen Creams. Ministry of Consumer Affairs. . 2013. [Google Scholar]
- Bachelet D, Barnes P, Brown D. Latitudinal And Seasonal Variation In Calculated Ultraviolet-B Irradiance For Rice-Growing Regions Of Asia. Photochem Photobiol. 1991;54(3):411-33. [Google Scholar]
- Brenner M, Hearing V. Review The Protective Role of Melanin Against UV Damage in Human Skin. Photochem Photobiol. 2008;84(3):539-88. [Google Scholar]
- Orazio J, Jarrett S, Ortiz A, Scott T. UV Radiation and the Skin. Int J Mol Sci. 2013;14(6):12222-70. [Google Scholar]
- Panich U, Sittithumcharee G, Rathviboon N, Jirawatnotai S. Ultraviolet Radiation-Induced Skin Aging : The Role of DNA Damage and Oxidative Stress in Epidermal Stem Cell Damage Mediated Skin Aging. Hindawi Publ Corp Stem Cells Int. 2016. [Google Scholar] [Crossref]
- Debuys H, Levy S, Murray J, Madey D, Pinnell S. Modern Approaches to Photoprotection. Dermatologic Asp Cosmet. 2000;18(4):577-90. [Google Scholar]
- Diffey B. Sunscreens as a preventative measure in melanoma : an evidence-based approach or the precautionary principle ?. Br J Dermatol. 2009;161(3):25-32. [Google Scholar]
- Diffey B. The impact of topical photoprotectants intended for daily use on lifetime ultraviolet exposure. J Cosmet Dermatol. 2008;10(3):245-50. [Google Scholar]
- Wijayanti L, Swasono T. Synthesis and evaluation of chalcone Derivatives as Novel Sunscreen agent. Molecules. 2021;26(9). [Google Scholar] [Crossref]
- Kelly J, Murphy J. Mitochondrial Tolerance to single and repeat exposure to simulated sunlight in human epidermal and dermal skin cells. J Photochem Photobiol. 2016;165:298-304. [Google Scholar] [Crossref]
- Lawrence K, Douki T, Herzog B, Young A. The UV/Visible Radiation Boundary Region (385–405 nm) Damages Skin Cells and Induces “dark” Cyclobutane Pyrimidine Dimers in Human Skin in vivo. Scientific Rep. 2018;8(12722):1-12. [Google Scholar]
- Day C, Marchalik R, Merlino G, Michael H. Mouse models of UV-induced melanoma : genetics , pathology , and clinical relevance. Lab Invest. 2017;97(6):698-705. [Google Scholar]
- Reddy P, kumar AS, Jain V. Sunscreens: Developments and challenges. Int J Appl Pharm. 2018;10(6). [Google Scholar] [Crossref]
- Dahabra L, Broadberry G, Gresley A, Najlah M. Sunscreens Containing Cyclodextrin Inclusion Complexes for Enhanced Efficiency : A Strategy for Skin Cancer Prevention. Molecules. 2021;26(6). [Google Scholar] [Crossref]
- Thi L, Tran N, Van V, Moon J, Park C. Recent Trends of Sunscreen Cosmetic : Cosmetics. Cosmetics. 2019;6(4):1-15. [Google Scholar]
- Trullas C, Miguel A, Paris C, Lhiaubet-Vallet V, Jime O, Valencia D. A Blocked Diketo Form of Avobenzone : Photostability , Photosensitizing Properties and Triplet Quenching by a Triazine-derived UVB-filter. Photochem Photobiol. 2009;85(1):178-84. [Google Scholar]
- Gaspar L, Campos P. Evaluation of the photostability of different UV filter combinations in a sunscreen. Int J Pharm. 2006;307(2):123-31. [Google Scholar]
- Kockler J, Oelgemöller M, Robertson S, Glass B. Influence of Titanium Dioxide Particle Size on the Photostability of the Chemical UV-Filters Butyl Methoxy Dibenzoylmethane and Octocrylene in a Microemulsion. Cosmetics. 2014;1(2):128-39. [Google Scholar]
- Sabzevari N, Mba S, Norton S, Fivenson D. International Journal of Women ’ s Dermatology Sunscreens : UV filters to protect us : Part 1 : Changing regulations and choices for optimal sun protection. Int J Women’s Dermatol. 2021;7(1):28-44. [Google Scholar]
- Smijs STG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens : focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95-112. [Google Scholar] [Crossref]
- Bedosky L. The Difference Between Chemical and Mineral Sunscreen. Everyday Health. . 2021. [Google Scholar]
- Dellorto D. Avoid sunscreens with potentially harmful ingredients, group warns [Internet]. CNN health. . 2012. [Google Scholar]
- . Sunblock Ingredients - Fuel on Your Face?. . . [Google Scholar]
- Sun S, Lim S, Kim M, Hwa K, Baek S. Risk assessment of ethylhexyl dimethyl PABA in sunscreen cosmetic products. Toxicol Lett. 2017;280(2):105-11. [Google Scholar]
- Siller A, Blaszak S. Update About the Effects of the Sunscreen Ingredients Oxybenzone and Octinoxate on Humans and the Environment. Plast Surg Nurcing. 2018;38(4):158-61. [Google Scholar]
- Madan R, Levitt J. A review of toxicity from topical salicylic acid preparations. J Am Acad Dermatol. 2014;70(4):788-92. [Google Scholar]
- Schlumpf M, Cotton B, Conscience M, Haller V, Steinmann B, Lichtensteiger W. In vitro and in vivo estrogenicity of UV screens. Environ Health Perspect. 2001;109(3):239-44. [Google Scholar]
- Palm M. Update on photoprotection. Dermatol Ther. 2007;20(5):360-76. [Google Scholar]
- Cardillo D, Sencadas V, Devers T, Islam M, Tehei M, Rosenfeld M. Attenuation of UV absorption by poly(lactic acid)-iron oxide nanocomposite particles and their potential application in sunscreens. Chem Eng J [Internet]. 2020;405(1). [Google Scholar] [Crossref]
- Newman M, Stotland M, Ellis J. The safety of nanosized particles in titanium dioxide e and zinc oxide e based sunscreens. J Am Dermatol. 2009;61(4):685-92. [Google Scholar]
- Saewan N, Jimtaisong A. Natural products as photoprotection. J Cosmet Dermatol. 2015;14(1):1-17. [Google Scholar]
- Anitha D, Reddy K, Venkatesh P, Raani M. A Review - Herbal Sunscreen Agents On Skin Protection.. Eur J Pharm Med Res. 2016;3(11):308-21. [Google Scholar]
- Rasheed A, Shama S, Mohanalakshmi S, Ravichandran V. Formulation, characterization and in vitro evaluation of herbal sunscreen lotion. Orient Pharm Exp Med. 2012;12(4):241-6. [Google Scholar]
- Aburjai T, Natsheh F. Plants Used in Cosmetics. Phyther Res. 2003;17(9):987-1000. [Google Scholar]
- Chanchal D, Swarnlata S. Novel approaches in herbal cosmetics. J Cosmet Dermatol. 2008;7(2):89-95. [Google Scholar]
- Singh A, Kalaivani M, Chaudhary P, Srivastava S, Goyal K, Gupta R. Opportunities and Challenges in Development of Phytopharmaceutical Drug in India- A SWOT Analysis. J Young Pharm. 2019;11(3):322-9. [Google Scholar]
- Murch S, Krishnaraj S. Phytopharmaceuticals: Problems, limitations, and solutions. Sci Rev Altern Med. 2000;4(2):33-7. [Google Scholar]
- . The Advantages And Disadvantages of Herbal Drugs Biology Essay. . . [Google Scholar]
- Rasheed A, Shama N, Veerasamy R. Characterization and in vitro evaluation of herbal sunscreen lotion Formulation. Orient Pharm Exp Med. 2012;12:241-6. [Google Scholar]
- Bambal V, Wyawahare N, Turaskar A, Mishra M. Study of Sunscreen Activity of Herbal Cream Containing Flower Extract of Nyctanthes Arbortristis L.. Int J Pharm Sci Rev Res. 2011;11(1):142-8. [Google Scholar]
- Marodkar R. Herbal Sunscreen Gel with Microbeads Technology Herbal Sunscreen Gel with Microbeads Technology. Int J Adv Study Res Work. 2020;3(3):1-8. [Google Scholar]
- Acharya R, Priya A, Mehta J. Bhawna Sharma PMJ. Formulation of Herbal Gel From Hydroalcoholic Extract of Eclipta Alba (L.). Career Int J Sci Technol. 2018;1(2):13-5. [Google Scholar]
- Shweta K, Swarnlata S. Efficacy Study of Sunscreens Containing Various Herbs for Pro - tecting Skin from UVA and UVB Sunrays. Pharmacognosy Mag. 2009;4(19):238-86. [Google Scholar]
- Vecino X, Cruz J, Moldes A. Design and characterization of greener sunscreen formulations based on mica powder and a biosurfactant extract. Powder Technol. 2017;327:442-8. [Google Scholar] [Crossref]
- Taylor P, Masuma R, Okuno T, Shahabuddin M, Choudhuri K, Saito T. Effect of Tinospora cordifolia on the reduction of ultraviolet radiation - induced cytotoxicity and DNA damage in PC12 cells Effect of Tinospora cordifolia on the reduction of ultraviolet radiation-in. J Environ Sci Heal. 2014;49(6):416-21. [Google Scholar]
- Sahu M. Preparation and Assessment of Sunscreen Cream Containing Extract Acquired from Plant Origin. J Pharm Sci Emerg Drugs. 2019;7(1). [Google Scholar] [Crossref]
- Dinanath G, Namdeo J. Development of stable emulsified formulations of Terminalia arjuna for topical application: evaluation of antioxidant activity of final product and molecular docking study. Drug Develop Industrial Pharm. 2019;45(11):1740-5. [Google Scholar]
- Saraf S, Kaur C. Phytoconstituents as photoprotective novel cosmetic formulations. Pharmacogn Rev. 2010;4(7):1-11. [Google Scholar]
- Salvi V, Pawar P. Nanostructured lipid carriers ( NLC ) system : A novel drug targeting carrier. J Drug Deliv Sci Technol. 2019;51:255-67. [Google Scholar] [Crossref]
- Sanad R, Abdelmalak N, Tahany S, Badawi AA. Formulation of a Novel Oxybenzone-Loaded Nanostructured Lipid Carriers ( NLCs ). AAPS Pharm SciTech. 2010;11(4):1684-94. [Google Scholar]
- Radika P, Nanocapsules T. A New Approach in Drug Delivery. Int J Pharm Sci Res. 2011;2(6):1426-35. [Google Scholar]
- Barre G. Biodegradable polymer nanocapsules containing a sunscreen agent : preparation and photoprotection. Eur J Pharm Biopharm. 2001;52(2):191-6. [Google Scholar]
- Khan I, Saeed K, Khan I. Nanoparticles : Properties , applications and toxicities. Arab J Chem. 2017;12(7):908-31. [Google Scholar]
- Frizzo M, Emilio P, Berres P, Júnior E, Campos C, Costa C. Simultaneous encapsulation of zinc oxide and octocrylene in poly ( methyl methacrylate-co-styrene ) nanoparticles obtained by miniemulsion polymerization for use in sunscreen formulations. Colloids Surfaces A [Internet]. 2018;561(20):39-46. [Google Scholar]
- Patel H, Patel U, Shah C, Akbari B. Formulation and Development of Nanosuspension as an Alternative Approach for Solubility and Dissolution Enhancement of Aceclofenac. Int J Adv Pharm. 2018;7(5):33-47. [Google Scholar]
- Muller C. In vitro erythemal UV-A protection factors of inorganic sunscreens distributed in aqueous media using carnauba wax - decyl oleate nanoparticles. Eur J Pharm Biopharm. 2007;65(1):122-7. [Google Scholar]
- Yadav P, Khailar P, Pradesh U. Solid Lipid Nanoparticles: An effective and [romising drug delivery system- A Review. Int J Pharm Sci Res. 2014;5(4):1152-62. [Google Scholar]
- Rodrigues L, Jose J. Exploring the photo protective potential of solid lipid nanoparticle-based sunscreen cream containing Aloe vera. Environ Sci Poluuution Res Int. 2020;17:20876-88. [Google Scholar]
- Jaiswal M, Dudhe R, Sharma P. Nanoemulsion : an advanced mode of drug delivery system. 3 Biotech. 2015;5(2):123-7. [Google Scholar]
- Hamid K, Wais M, Sawant G. A Review On Nanoemulsions : Formulation , Composition , And Applications.. Asian J Pharm Clin Res. 2021;14(4):1-7. [Google Scholar]
- Suhendi A, Muhtadi M. Gel Nanoemulsion of Rambutan Fruit peel extracts: Formulation, Physical Properties, Sunscreen Protecting, and Antioxidant Activity. Asian J Pharm Clin Res. 2017;10(11). [Google Scholar] [Crossref]
- Bhalke R, Kulkarni S, Kendre P, Pande V, Giri M. A facile approach to fabrication and characterization of novel herbal microemulsion-based UV shielding cream. Futur J Pharm Sci. 2020;6(76):1-10. [Google Scholar]
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo S, Zarghami N. Liposome : classification , preparation , and applications. Nanoscale Res Lett. 2013;8(1). [Google Scholar]
- Liu D, Haiyang H, Zhixiu L. Quercetin deformable liposome: Preparation and efficacy against ultraviolet B induced skin damages in vitro and in vivo. J Photochem Photobiol B Biol. 2013;127:8-17. [Google Scholar]
- Bhattacharya S. Phytosomes: The New Technology for Enhancement of Bioavailability of Botanical and Nutraceuticals. Int J Heal Res. 2009;2(3):223-55. [Google Scholar]
- Dian A, Nurul R, Courtenay A, Manggau M, Donnelly R, Rahman L. Phytosomal nanocarriers as platforms for improved delivery of natural antioxidant and photoprotective compounds in propolis : An approach for enhanced both dissolution behaviour in biorelevant media a. J Photochem Photobiol B Biol [Internet]. 2019;205. [Google Scholar] [Crossref]
- Avadhani KS, Manikkath J, Tiwari M, Godavarthi A, Vidya S, Raghu C. Skin delivery of epigallocatechin-3-gallate ( EGCG ) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage loaded nano-transfersomes for antioxidant and anti-aging effects. Drug Deliv. 2017;24(1):61-74. [Google Scholar]
- Geoffrey K, Mwangi A, Maru S. Sunscreen products : Rationale for use , formulation development and regulatory considerations. Saudi Pharm J [Internet]. 2019;27(7):1009-27. [Google Scholar]
- Abstract
- Introduction
- Sunscreens
- Literature Review on Herbal Sunscreen Formulations
- Recent Trends in Sunscreen Deliveries
- Nano lipid carrier (NLC)
- Nano-capsules
- Nanoparticles
- Nanosuspension
- Solid lipid nanoparticles (SLN)
- Nanoemulsion
- Microemulsion
- Liposomes
- Phytosomes
- Transferosomes
- Regulatory Guidelines for Labelling of Sunscreens
- Abbreviations
- Source of Funding
- Conflict of Interest
- References
How to Cite This Article
Vancouver
Shah Y, Mewada R. Recent advances in sunscreen agents and their formulations: A review [Internet]. Int J Pharm Chem Anal. 2022 [cited 2025 Oct 11];9(4):141-150. Available from: https://doi.org/10.18231/j.ijpca.2022.027
APA
Shah, Y., Mewada, R. (2022). Recent advances in sunscreen agents and their formulations: A review. Int J Pharm Chem Anal, 9(4), 141-150. https://doi.org/10.18231/j.ijpca.2022.027
MLA
Shah, Yamini, Mewada, Rajvee. "Recent advances in sunscreen agents and their formulations: A review." Int J Pharm Chem Anal, vol. 9, no. 4, 2022, pp. 141-150. https://doi.org/10.18231/j.ijpca.2022.027
Chicago
Shah, Y., Mewada, R.. "Recent advances in sunscreen agents and their formulations: A review." Int J Pharm Chem Anal 9, no. 4 (2022): 141-150. https://doi.org/10.18231/j.ijpca.2022.027
